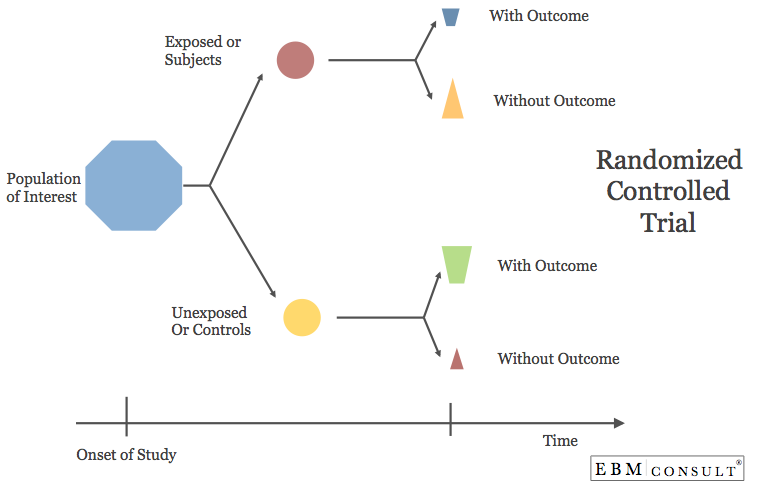
Randomized Controlled Trial (RCT)
|
|---|
- A prospective study where patients from a population of interest are randomly assigned to either a experimental (treatment) or a control group and then are followed up a specific time intervals to collect data on the outcomes of interest.
- Patients in the control group usually receive a placebo or comparative treatment generally considered already part of the standard of care.
- Considered the gold standard for helping to explain an effect
- When appropriate randomization has occurred, this design allows for washout of most population bias
- Reduced influence by confounders because they can be controlled for by the investigators
- Reduced variability in the outcome(s) being measured, thus
increasing the precision of estimation (assumes proper control of confounders)
- Easier to blind patients than observational studies
Description
Advantages