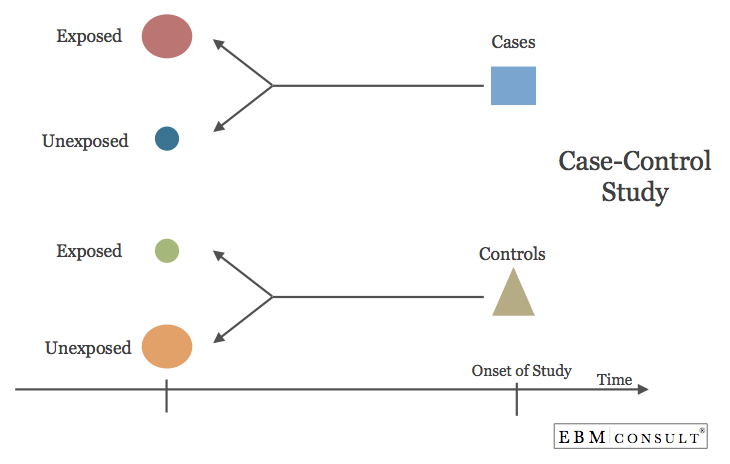
Case-Control Study
|
|---|
- A study design where the investigator identifies and selects patients who have the endpoint or outcome of interest (i.e., "cases") and also patients without the endpoint or outcome of interest (i.e., "controls") and looks back in time to identify exposures or characteristics that are linked to the cases.
- Case-control studies are retrospective.
- Less expensive
- Easier to do and take less time compared to most prospective studies
- Can be useful when obtaining follow-up data that is difficult to obtain due to the nature of population being studied.
- When disease being studies is either rare or when there is
a long period of time between exposure to the time of manifesting the outcome,
this study design can be more efficient.
- Depending on the exposure and outcome of interest, this design may be the only ethical way to evaluate something.
- Potential recall bias
- Subject to selection bias
- Generally do not allow investigators to calculate an incidence or absolute risk
- Case-control studies rank below systematic reviews, metal-analysis, randomized controlled trials, and cohort studies, but are above cross-sectional surveys and case reports.
- Oxford Centre of Evidence-Based Medicine give it a level of evidence = 3a/b to 4 (depending on the quality of studies)
- A case-control study may be able to show an association between an exposure and outcome, but it cannot explain causation.
- DiPietro NA. Methods in epidemiology: observational study designs. Pharmacotherapy 2010;30(10):973-84.
- Noordij M et al. Study designs in clinical research. Nephron Clin Pract 2009;113(3):c218-21.
- Guyatt GH, et al. Users' guides to the medical literature. IX. A method for grading health care recommendations. JAMA 1995; 274:1800-4.
- Oxford Centre for Evidenced-Based Medicine. Levels of Evidence. March 2009. CEBM
Description of a Case-Control Study
Advantages
Disadvantages
Considerations
References