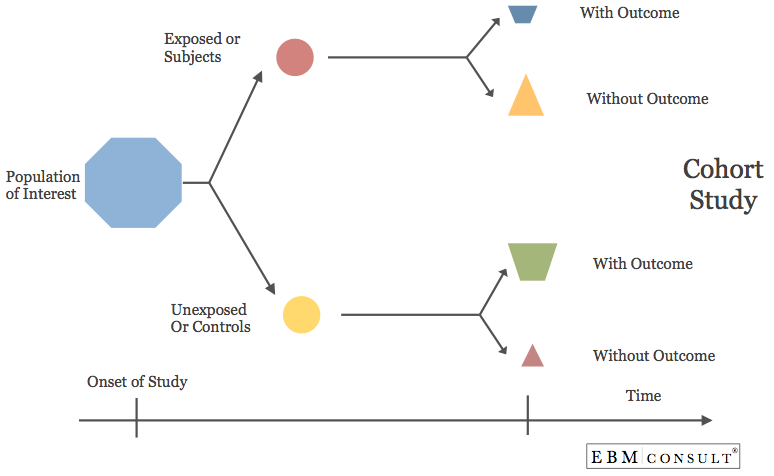
Cohort Study: Biostatistics Overview
|
|---|
- A study design that identifies and selects two groups of patients out of a population of interest and places them into one of two cohorts, one cohort who are exposed to an intervention and another cohort have not been exposed that intervention. They are then followed over time to see if they develop the outcome of interest at various time points.
- Cohort studies are almost always prospective, but some can
be retrospective cohort studies.
- Retrospective cohort studies are also called historical cohort studies and can evaluate a medical event from a time point in the past that then evaluates data up to the present.
- Can more clearly show the time of exposure and development of the outcome because the subjects are without the disease at baseline.
- Allows for evaluation of more than one outcome as it relates to an exposure
- Allows for the calculation of the incidence
- Helpful when needing to evaluate rare exposures
- Can be expensive and time consuming because of needing to follow a large number of people
- Loss of follow up can begin to introduce bias
- May not be good for rare diseases
- For retrospective cohort studies:
- Recall bias due to reliance on memory of the subjects
- The quality of data collected or available from the past
- Case-control studies rank below systematic reviews, metal-analysis,
and randomized controlled trials, but are above case-control studies, cross-sectional surveys, and case reports.
- Oxford Centre of Evidence-Based Medicine give it a level of evidence = 2b to 4 (depending on the quality of studies)
- There are many well-known cohort studies that have contributed to medicine and include:
- British Doctors' Cohort Study
- Framingham Heart Study
- Nurses' Health Study
- Physicians' Health Study
- Women's Health Initiative
- DiPietro NA. Methods in epidemiology: observational study designs. Pharmacotherapy 2010;30(10):973-84.
- Noordij M et al. Study designs in clinical research. Nephron Clin Pract 2009;113(3):c218-21.
- Guyatt GH, et al. Users' guides to the medical literature. IX. A method for grading health care recommendations. JAMA 1995; 274:1800-4.
- Oxford Centre for Evidenced-Based Medicine. Levels of Evidence. March 2009. CEBM
Description of a Cohort Study
Advantages
Disadvantages
Application
References