Nimodipine (Nymalize): Drug Monograph
|
|---|
- Improve neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH) from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I-V)
- General Notes:
- Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Give one hour before a meal or two hours after a meal.
- Do not administer intravenously or by other parenteral routes.
- Subarachnoid Hemorrhage:
- 20 mL (60 mg) orally every 4 hours starting within 96 hours of the onset of SAH and then continue for 21 consecutive days
- None
- Hypotension - monitor blood pressure
- Patients with cirrhosis: higher risk of adverse reactions. Monitor blood pressure and pulse.
- CYP3A4 strong inhibitors - may significantly increase risk of hypotension. Concomitant use should generally be avoided.
- CYP3A4 strong inducers - may significantly reduce efficacy of nimodipine. Concomitant use should generally be avoided.
- No reports of overdosage.
- Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral vasodilation with marked systemic hypotension.
- May require active cardiovascular support with pressor agents and specific treatments for calcium channel blocker overdose.
- Dialysis is not likely to be of benefit.
- Substrate: CYP3A4
- Inhibitor: None
- Inducer: None
- Other:
- Anti-Hypertensives - may increase risk of hypotension. Monitor blood pressure.
- CYP3A4 moderate and weak inhibitors - may increase risk of hypotension. Monitor blood pressure. Dose reduction of nimodipine may be needed. Avoid grapefruit juice.
- CYP3A4 moderate and weak inducers - may reduce efficacy of nimodipine. Dose increase may be needed.
- Pregnancy: Pregnancy Category C.
- Labor and Delivery: None
- Nursing Mothers: It is not known whether the drug is excreted in human milk. Decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
- Renal Impairment: None
- Hepatic Impairment: Dosage adjustment for patients with cirrhosis.
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: Dosing in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
- It is not known whether the drug is excreted in human milk. Decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
-
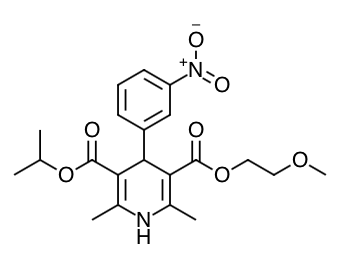
Scientific Name: isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl- 4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
-
Empirical Formula: C21H26N2O7
-
Molecular Weight: 418.5
- Nimodipine is a dihydropyridine calcium channel blocker.
- Nimodipine inhibits calcium ion transfer into smooth muscle cells and thus inhibits contractions of vascular smooth muscle.
- In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood-brain barrier; concentrations of nimodipine as high as 12.5 ng/mL have been detected in the cerebrospinal fluid of nimodipine-treated SAH patients.
- The precise mechanism of action of nimodipine in reducing the incidence and severity of ischemic deficits in adult patients with SAH from ruptured intracranial berry aneurysms is unknown. Although the clinical studies demonstrate a favorable effect of nimodipine on the severity of neurological deficits caused by cerebral vasospasm following SAH, there is no arteriographic evidence that nimodipine either prevents or relieves the spasm of these arteries. However, whether or not the arteriographic methodology utilized was adequate to detect a clinically meaningful effect, if any, on vasospasm is unknown.
- Absorption:
- Rapidly absorbed after oral administration, and peak concentrations are generally attained within one hour.
- Distribution:
- Nimodipine is over
95% bound to plasma proteins
- Metabolism:
- The metabolism of nimodipine is mediated by CYP3A4. Because of a high first-pass metabolism, the bioavailability of nimodipine averages 13% after oral administration.
- Elimination:
- The terminal elimination half-life is approximately 8 to 9 hours but earlier elimination rates are much more rapid, equivalent to a half-life of 1-2 hours
- Nimodipine is eliminated almost exclusively in the form of metabolites and less than 1% is recovered in the urine as unchanged drug.
- Food Effects:
- In a study of 24 healthy male volunteers, administration of nimodipine capsules following a standard breakfast resulted in a 68% lower peak plasma concentration and 38% lower bioavailability relative to dosing under fasted conditions.
- Patients with Cirrhosis:
- The bioavailability of nimodipine is significantly increased in patients with cirrhosis; with Cmax approximately double that in normals, which necessitates lowering the dose in this group of patients.
- Geriatric Patients:
- In a single parallel-group study involving 24 elderly subjects (aged 59-79) and 24 younger subjects (aged 22-40), the observed AUC and Cmax of nimodipine was approximately 2-fold higher in the elderly population compared to the younger study subjects following oral administration (given as a single dose of 30 mg and dosed to steady-state with 30 mg three times daily for 6 days). The clinical response to these age-related pharmacokinetic differences, however, was not considered significant.
- Inform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure. Inform them that use of nimodipine with anti-hypertensives can cause increased drop in blood pressure.
- Patients should be aware that ingestion of grapefruit or grapefruit juice should be avoided when taking nimodipine due to its ability to increase nimodipine plasma concentrations and potential to increase the risk of hypotension.
- Pregnant women should be advised that a harmful effect of nimodipine on the fetus cannot be ruled out and the drug should only be used if the potential benefit justifies the potential risk to the fetus.
- EBM Focused Topic: Nimodipine Use for Subarachnoid Hemorrhage
- EBM Focused Topic: Endovascular Treatment for Acute Ischemic Stroke
- Anatomy: Subdural vs Epidural Hematoma
- Anatomy: Homunculus (Sensory & Motor)
- Anatomy: Bells Palsy vs Stroke
- Drug Monograph: Amlodipine (Norvasc)
- Drug Monograph: Felodipine (Plendil)
- Drug Monograph: Amiodarone (Cordarone; Pacerone)
Indications
Dosing
(Adult)
(Pediatrics)
Warnings
Overdose
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
Other EBM Consult Related Content
MESH Terms & Keywords
|
|---|
|

