-
Meperidine
(pethidine, Demerol) is a well known opioid analgesic that has been used in
practice since the early 1940's. Unfortunately, meperidine is also known
to induce seizures from the accumulation of meperidine's metabolite,
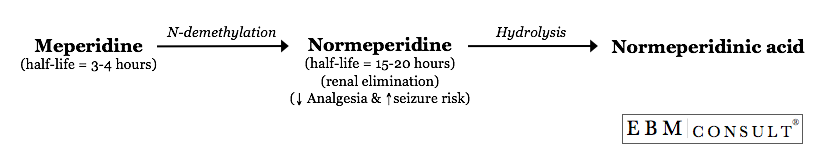
normeperidine (also called norpethidine).1-3 When meperidine is
administered, it undergoes extensive hepatic metabolism via N-demethylation to
produce normeperidine.2 Normeperidine has a half-life of 15-20 hours
which is 5-10 times greater than the half-life of the parent compound,
meperidine.2,3 Therefore, when meperidine is administered on a scheduled
basis, normeperidine can easily accumulate. This is especially true in
patients with renal impairment since normeperidine is primarily renally
eliminated.4 In addition, meperidine rapidly appears in the cerebrospinal
fluid (CSF) while normeperidine appears gradually and erratically in the CSF.5
The CSF penetration is important because normeperidine has half the analgesic
potency as compared to meperidine but produces twice the
potency as a proconvulsant compared with meperidine.6
See the figure below for the basic metabolism of meperidine.7
How
does a patient exhibit signs of normeperidine toxicity?
Accumulation of normeperidine will produce central nervous system (CNS) effects
such as mood alterations, anxiety, tremor, myoclonus, and seizures.8
The tremors, myoclonus, and seizures correlate with the plasma
normeperidine concentrations rather than meperidine plasma concentrations.3 Patients
experiencing myoclonus and/or seizures display a mean normeperidine plasma
concentration of 814ng/mL, which is approximately twice the level in those
experiencing only tremors, shaking, or twitching sensations.3Electroencephalograms
in patients exhibiting CNS effects also demonstrate a diffuse, slow-wave
activity with sporadic paroxysmal activity.3 In addition, the development
of agitated delirium generally precedes the development of seizures.3
Unfortunately, in some reports of patients receiving patient controlled
analgesia with meperidine, seizures occurred without the classic warning signs
of myoclonus and agitation.9 For example, one study found that in
over 400 samples, the median normeperidine concentrations were 303, 519, and
640 ng/mL for those patients exhibiting no symptoms, mild symptoms, and
seizures, respectively.9 However there is a wide interpatient
variability in the presence of symptoms and the levels. It has been
suggested that if a patient exhibits signs or symptoms of normeperidine
toxicity, it can be assumed that the plasma normeperidine level will be
elevated > 50 ng/mL and meperidine should be discontinued.9 Since the
normeperidine half-life is 15-20 hours, the neurological signs will slowly
resolve (up to 5 days after the meperidine is stopped) and do not correlate
with any other chemistry blood values.2,3
We
have reviewed the causative role of normeperidine and seizures, but what is the
exact mechanism? The exact mechanism of how
normeperidine induces seizures is unknown. It has been determined from a
rat model study that the seizures are not dopaminergic or cholinergic in
nature.10 However, meperidine has the ability to increase serotonin by
blocking its neuronal uptake.2,11 The use of meperidine in combination
with other agents known to increase serotonin has been linked with the
development of serotonin syndrome.12-14 Serotonin syndrome is
characterized by the excitatory effects (e.g., delirium, rigidity, convulsions)
similar to normeperidine toxicity.14 Since meperidine is considered to be
a weak serotonin reuptake inhibitor, its potential to induce a serotonin
syndrome alone is uncommon.14
What
patients are at risk for meperidine induced seizures?
Due to the above information, many well known professional societies and
accrediting bodies have taken positions against the general use of
meperidine. For example, the authors of the 2008 National Comprehensive
Cancer Network (NCCN) guidelines on cancer pain management do not
recommend the use of meperidine in cancer patients with pain.18
In addition, the American College of Surgeons (ACS) distinctively
cautions against meperidine.19 Several national health care
organizations such as the Centers for Medicare and Medicaid Services (CMS),
Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and
Agency for Health Care Policy and Research or currently named the Agency for
Healthcare Research and Quality (AHRQ) also do not recommend meperidine for
pain management.20-22
References:
- Beckwith SK Hospice, cancer pain management, and symptom control. In:Weiner's Pain Management: A Practical Guide for Clinicians, 7th ed. Taylor & Francis Group, LLC, Boca Raton, FL, 2006:1327-1352.
- Gutstein HB, Akil H. Opioid analgesics. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman and Gilman's The Pharmacological Basis of Therapeutics, 10th ed. McGraw-Hill. New York, NY, 2001: 569-619.
- Kaiko
RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory
effects of meperidine in cancer patients. Ann Neurol 1983;13(2):180-5.
- Szeto
HH, Inturrisi CE, Houde R, et al. Accumulation of normeperidine, an
active metabolite of meperidine, in patients with renal failure of
cancer. Ann Intern Med 1977;86(6):738-41.
- Boréus
LO, Sköldefors E, Ehrnebo M. Appearance of pethidine and norpethidine
in cerebrospinal fluid of man following intramuscular injection of
pethidine. Acta Anaesthesiol Scand 1983;27(3):222-5.
- Kornitzer
BS, Manace LC, Fischberg DJ, Leipzig RM. Prevalence of meperidine use
in older surgical patients. Arch Surg 2006;141(1):76-81.
- Mather LE, Gourlay GK. Biotransformation of opioids: significance for pain therapy. In: Nimmo WS, Smith G, eds. Opioid Agonist/Antagonist Drugs in Clinical Practice. Amsterdam: Excerpta Medica, 1984;31-46.
- Seifert
CF, Kennedy S. Meperidine is alive and well in the new millennium:
evaluation of meperidine usage patterns and frequency of adverse drug
reactions. Pharmacotherapy 2004;24(6):776-83.
- Plummer JL, Gourlay GK, Cherry DA. Norpethidine toxicity. Pain Reviews 2001;8(3-4):159-70.
- Plummer
JL, Gourlay GK, Cmielewski PL, Odontiadis J, Harvey I. Behavioural
effects of norpethidine, a metabolite of pethidine, in rats. Toxicology
1995;95(1-3):37-44.
- Latta KS, Ginsberg B, Barkin RL. Meperidine: a critical review. Am J Ther 2002;9(1):53-68.
- Altman EM, Manos GH. Serotonin syndrome associated with citalopram and meperidine. Psychosomatics 2007;48(4):361-3.
- Huang
SS, Jou SH, Chiu NY. Catatonia associated with coadministration of
tramadol and meperidine. J Formos Med Assoc 2007;106(4):323-6.
- Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth 2005;95(4):434-41.
- Danziger
LH, Martin SJ, Blum RA. Central nervous system toxicity associated with
meperidine use in hepatic disease. Pharmacotherapy 1994;14(2):235-8.
- Simopoulos
TT, Smith HS, Peeters-Asdourian C, Stevens DS. Use of meperidine in
patient-controlled analgesia and the development of a normeperidine
toxic reaction. Arch Surg 2002;137(1):84-8.
- Umans
JG, Inturrisi CE. Antinociceptive activity and toxicity of meperidine
and normeperidine in mice. J Pharmacol Exp Ther 1982;223(1):203-6.PubMed
- NCCN Guidelines.http://www.nccn.org/professionals/physician_gls/PDF/pain.pdf. Accessed 11/30/2008.
- Wilmore DW, Cheung LY, Harken AH, Holcroft JW, Meakins JL, Soper NJ, eds. In: ACS Surgery: Principles and Practice. New York, NY: WebMD Corp; 2002.
- Joint
Commission on Accreditation of Healthcare Organizations. Improving the
Quality of Pain Management through Measurement and Action. Oakbrook
Terrace, Ill: Joint Commission Resources; 2003.
- Fick DM, Cooper
JW, Wade WE, et al. Updating the Beers criteria for potentially
inappropriate medication use in older adults: results of a US consensus
panel of experts. Arch Intern Med 2003; 163(22):2716-24.
- Clinical
Practice Guideline Number 1: Acute Pain Management: Operative or
Medical Procedures and Trauma. Clinical Practice Guideline.
Rockville, Md: Agency for Health Care Policy and Research, US Dept of
Health and Human Services;1992. AHCPR publication 92-0032.
|


