Ethylene Glycol Toxicology - An Overview
Summary:
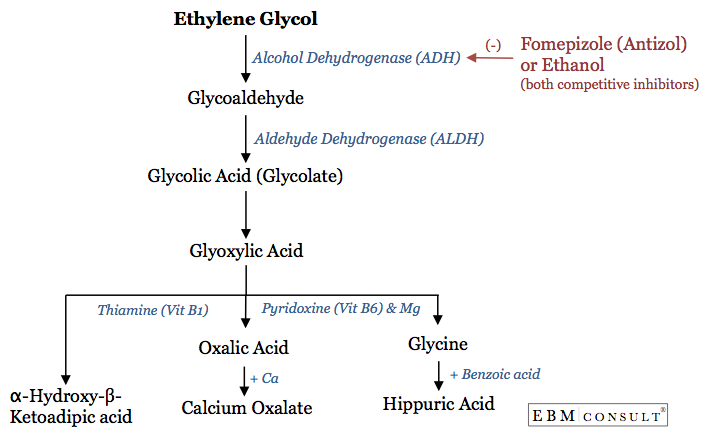
- Ethylene glycol toxicity is important to recognize because of its conversion to acid metabolites that can cause a metabolic acidosis and renal toxicity.
- Treatment is multifactorial, but if implemented early can prevent some of these complications.
Editor: Anthony J. Busti, MD, PharmD, FNLA, FAHA
Last Reviewed: August 2015
Ethylene Glycol Toxicology
|
|---|
- Conversion Factor = 6.2
- How to estimate methanol concentration (while waiting for actual level):
- Step 1: Calculate Osm = (2 x Na) + (BUN/2.8) + (Glucose/18) + (Ethanol level/4.6)
- Step 2: Determine Osm Gap = (Measured Osm) - (Calculated Osm)
- If Gap > 15 = abnormal
- If Gap > 25 = very high --> give empiric fomepizole (Antizol)
- Step 3: Estimate Ethylene Glycol Concentration (mg/dL) = 6.2 x Osm gap
- General Notes:
- Call or consult with Poison Center 1-800-222-1222
- The half-life of the parent compound (ethylene glycol) changed based on situation.
- No Treatment = 8.5 hrs
- Fomepizole Treatment = 14 - 17 hrs with normal renal function & ~ 50 hrs with poor renal function
- Hemodialysis = 2.5 hrs
- GI Decontamination:
- Generally avoided
- Rarely helpful unless very early on (within 15-30 min) since alcohols are rapidly absorbed or co-ingestions and airway is protected
- Fomepizole:
- Competes with ethylene glycol for metabolism via alcohol dehydrogenase (ADH) enzyme. Fomepizole has an 8,000 fold greater binding affinity to ADH and thus prevents the metabolism of ethylene glycol to toxic acid metabolites.
- Indications:
- Ethylene glycol levels > 20 mg/dL
- Reports of ingestion & any 2 of the following (osmol gap > 10-15 mOsm; arterial pH < 7.3, serum HCO3 < 20, UA showing calcium oxalate crystals)
- Dosing:
- 15 mg/kg IV x 1 dose, then 10 mg/kg every 12 hours x 4 doses, then 15 mg/kg every 12 hours until ethylene glycol level < 20 mg/dL and/or pH improves
- Can be irritating to the veins so dilute in 100 mL of 0.9% NS or D5W
- If getting HD, will need to redose every 4 hours since its low volume of distribution and degree of protein binding allows it to be removed easily.
- Also give thiamine and pyridoxine (see below)
- Thiamine:
- Facilitates the metabolism of one of the toxic acid metabolites, glycoxylic acid
- Dose: 100 mg IV daily until symptoms or acidosis resolve
- Vitamin B6 (Pyridoxine):
- Facilitates the metabolism of one of the toxic acid metabolites, glycoxylic acid
- Dose: 10 - 25 mg IV daily (no established dose)
- Sodium Bicarbonate:
- Use if pH < 7.3 (goal: 7.35 - 7.45)
- Dose: 150 mEq in 1 L of D5W and infuse at 150-200 mL/hr
- Note: Can lower calcium & potassium, so monitor
- Hemodialysis:
- Indications:
- pH < 7.25
- Renal failure electrolyte changes not responding to treatment
- Changes in vision
- Hemodynamic instability
- Toxic alcohol levels > 50 mg/dL
- General Notes:
- If getting fomepizole, re-dose every 4 hours due to increased elimination
- Ethylene glycol can interact with the assays that measure lactic acid, thereby causing the lactic acid to be higher than actually present.
- As ethylene glycol is metabolized the osmol gap will decrease, but the anion gap will increase (unless fomepizole is used).
- While this should not be relief upon for ruling in or out the presence of ethylene glycol toxicity, antifreeze contains fluorescein which can be easily seen under a Wood's lamp in present in vomit or the urine.
- Don't forget to monitor for calcium levels as it can decrease due to binding of oxalic acid and thus result in hypocalcemia.
- Toxicology: Isopropanol - Overview
- Toxicology: Methanol - Overview
- Pharmacology: Propylene Glycol Containing IV Medications
- Differential Diagnosis: Anion Gap (Elevated)
- Differential Diagnosis: Osmolar Gap (Elevated)
- Calculator: Anion Gap
Conversion Factor
Treatment
Clinical Considerations
Related Content