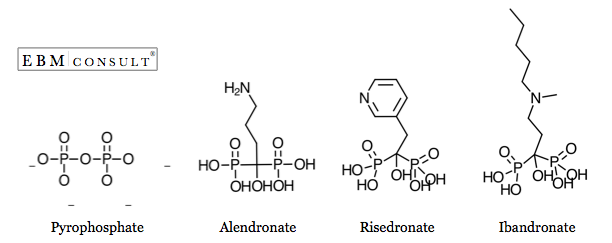
Bisphosphonates
are synthetic analogs of a naturally occurring compound called pyrophosphate
and are currently approved for the treatment of osteoporosis and other
metabolic bone disorders.(1,2) They work by binding to the bone matrix and
inhibiting osteoclast activity, either through direct induction of apoptosis of
osteoclasts or inhibiting the proteins osteoclasts require for their resorptive
effects.(1) Their chemical structure contains two phosphate groups and two side
chains (R1 and R2) bound to a central carbon (See figure). The side chains
affect bisphosphonate skeletal binding and prevent enzymatic breakdown in the
gastrointestinal tract by phosphatases. Despite this physiochemical advantage,
the oral bioavailability of bisphosphonates remains low at 0.6% to 1.5% of the
administered dose.(2,3)
In general, a drug may be absorbed
through the intestine by either the transcellular or paracellular route depending
on its physiochemical properties. The transcellular route involves passive
diffusion, carrier-mediated transport, and endocytosis; all of which require
the drug to be small and lipophilic. The paracellular route requires a drug to
be resistant to enzymatic degradation, small, and hydrophilic. Once a drug
passes through the epithelial cell layer of the gastrointestinal tract, it is
then absorbed into circulation to exert its effects.(4,5,6)
Bisphosphonates
are large, hydrophilic drugs, which prevents diffusion across the
gastrointestinal epithelium through the transcellular route.(7) The molecular
weight of alendronate is 249 grams/mole and its octanol/buffer partition
coefficient is 0.0017 independent of pH, all properties indicating a large,
hydrophilic drug. This leaves the paracellular route as a means for
bisphosphonates to be absorbed; however, their large size hinders their
movement through tight junctions in the gastrointestinal epithelium.
Additionally, the brush-border membrane is negatively charged and will often
repel the negatively charged phosphate groups on the bisphosphonate from the
epithelium and tight junctions. Finally, these negative charges also give
bisphosphonates the tendency to bind with cations such as calcium and magnesium
present in the intestinal lumen.8 In relation to this, the opening and closing
of tight junctions has been linked to shifts in intracellular and extracellular
movements of calcium. This additional supply of calcium ions may also interfere
with the movement of the bisphosphonate through these junctions.(6,8,9)
- Friedman
PA. Chapter 44. Agents Affecting Mineral Ion Homeostasis and Bone Turnover. In:
Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's: the
pharmacological basis of therapeutics. 12nd ed. New York: McGraw-Hill;
2011.
- Khosla
S, Bilezikian JP, Dempster DW, Lewiecki ME, Miller PD, Neer RM, Recker RR,
Shane E, Shoback D, and Potts JT. Benefits and risks of bisphosphonate therapy
for osteoporosis. J Clin Endocrinol Metab. 2012;97(7):2272-82.
- Cremers
S and Papapoulos. Pharmacology of bisphosphonates. Bone. 2011;49:42-49.
- Shargel
L, Wu-Pong S, Yu AB. Chapter 13. Physiologic Factors Related to Drug
Absorption. In: Shargel L, Wu-Pong S, Yu AB, eds. Applied biopharmaceutics
& pharmacokinetics. 6th ed. New York: McGraw-Hill; 2012.
- Barthe
L, Woodley J, and Houin G. Gastrointestinal absorption of drugs: methods and
studies. Fundam Clin Pharmacol. 1999;13:154-68.
- Hayashi
M and Tomita M. Mechanistic analysis for drug permeation through intestinal
membrane. Drug Metab Pharmacokinect. 2007;22(2):67-77.
- Porras
AH, Holland SD, and Gertz BJ. Pharmacokinetics of alendronate. Clin
Pharmacokinet. 1999;36(5):315-28.
- Lin
JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996.
18(2):75-85.
- Salama NN, Eddington ND, and Fasano A. Tight junction modulation
and its relationship to drug delivery. Adv Drug.


