Tigecycline (Tygacil): Drug Monograph
|
|---|
- Treatment of community-acquired bacterial pneumonia (CAP)
- Treatment of complicated intra-abdominal infections
- Treatment of complicated skin and skin structure infections caused by susceptible strains of indicated pathogens in patients ≥18 years of age
- Limitations of Use - (Not indicated for treatment of diabetic foot infection or hospital-acquired pneumonia, including ventilator-associated pneumonia)
- Community-Acquired Pneumonia (CAP):
- Initial: 100 mg IV over 30 - 60 min infusion
- Maintenance: 50 mg IV every 12 hours for 7-14 days
- Intra-Abdominal Infections (IAB; Complicated):
- Initial: 100 mg IV over 30 - 60 min infusion
- Maintenance: 50 mg IV every 12 hours for 5-14 days
- Skin and Skin Structure Infections (SSSI; Complicated):
- Initial: 100 mg IV over 30 - 60 min infusion
- Maintenance: 50 mg every 12 hours for 5-14 days
- 8-11 years:
- 1.2 mg/kg every 12 hours
- Maximum dose: 50 mg every 12 hours
- 12-17 years:
- 50 mg every 12 hours
- Do not use unless no alternative antibacterial drugs are available
- An increase in all-cause mortality has been observed in a meta-analysis of Phase 3 and 4 clinical trials in tigecycline-treated patients versus comparator.
- Tigecycline should be reserved for use in situations when alternative treatments are not suitable.
- All-cause mortality - a meta-analysis of Phase 3 and 4 clinical trials demonstrated an increase in all-cause mortality in tigecycline-treated patients compared to controls with a risk difference of 0.6% (95% CI 0.1, 1.2). The cause of this increase has not been established. An increase was also seen in a meta-analysis limited to the approved indications [0.6% (95% CI 0.0, 1.2)]. The greatest difference in mortality was seen in tigecycline-treated patients with ventilator-associated pneumonia.
- Anaphylaxis/anaphylactoid reactions - have been reported with use and may be life-threatening. Exercise caution in patients with known hypersensitivity to tetracyclines.
- Hepatic dysfunction and liver failure - have been reported with use.
- Pancreatitis, including fatalities - have been reported with use. If pancreatitis is suspected, then consider stopping treatment.
- Fetal harm - when administered to a pregnant woman.
- Tooth development - use of tigecycline during tooth development may cause permanent discoloration of the teeth.
- Clostridium difficile associated diarrhea - evaluate if diarrhea occurs
- Patients with intestinal perforation - caution should be exercised when considering tigecycline monotherapy in patients with complicated intra-abdominal infections (cIAI) secondary to clinically apparent intestinal perforation.
- Tetracycline-class effects - may include photosensitivity, pseudotumor cerebri and anti-anabolic action
- Superinfection - use may result in overgrowth of non-susceptible organisms, including fungi. Patients should be carefully monitored during therapy.
- Development of drug-resistant bacteria - prescribing tigecycline in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria
- No specific information is available on the treatment of overdosage.
- Signs and symptoms may include an increased incidence of nausea and vomiting.
- Tigecycline is not removed in significant quantities by hemodialysis.
- Warfarin - suitable anticoagulation test should be monitored with concomitant use
- Oral contraceptives - may render oral contraceptives less effective
- Pregnancy: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: It is not known whether this drug is excreted in human milk. Caution should be exercised when administered to a nursing woman.
- Renal Impairment: None
- Hepatic Impairment: No dosage adjustment is warranted in patients with mild to moderate impairment. Severe impairment (Child Pugh C): initial dose of 100 mg followed by 25 mg every 12 hours.
- Pediatric Patients: Use in patients under 18 years of age is not recommended. Safety and effectiveness in pediatric patients below the age of 18 years have not been established. Pediatric trials were not conducted because of the higher risk of mortality seen in adult trials.
- Geriatric Patients: No unexpected overall differences in safety or effectiveness were observed between elderly and younger subjects, but greater sensitivity to adverse events of some older individuals cannot be ruled out.
- It is not known whether this drug is excreted in human milk. Caution should be exercised when administered to a nursing woman.
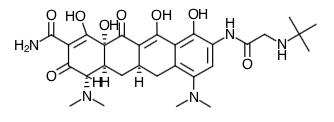
- Scientific Name: (4S,4aS,5aR,12aS)-9-[2-(tert-butylamino)acetamido]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide
- Empirical Formula: C29H39N5O8
- Molecular Weight: 585.65
- Cardiac Electrophysiology: No significant effect of a single intravenous dose of tigecycline 50 mg or 200 mg on QTc interval was detected in a randomized, placebo- and active-controlled four-arm crossover thorough QTc study of 46 healthy subjects.
- Absorption: Single dose (100 mg) Cmax = 1.45 mcg/mL (30 minutes infusion), 0.90 mcg/mL (60 minute infusion); AUC = 5.19 mcg•hr/mL. Multiple dose: (50 mg every 12 hours) Cmax =0.87 mcg/mL (30 minute infusion), 0.63 mcg/mL (60 minute infusion): AUC0-24 hr = 4.7 mcg•hr/mL
- Distribution:
- Protein Binding = 71 - 89%
- Volume of Distribution = 7 - 9 L/kg
- The in vitro plasma protein binding of tigecycline ranges from approximately 71% to 89% at concentrations observed in clinical studies (0.1 to 1.0 mcg/mL). The steady-state volume of distribution of tigecycline averaged 500 to 700 L (7 to 9 L/kg), indicating tigecycline is extensively distributed beyond the plasma volume and into the tissues. Following the administration of tigecycline 100 mg followed by 50 mg every 12 hours to 33 healthy volunteers, the tigecycline AUC0-12h (134 mcg∙h/mL) in alveolar cells was approximately 78-fold higher than the AUC0-12h in the serum, and the AUC0-12h (2.28 mcg∙h/mL) in epithelial lining fluid was approximately 32% higher than the AUC0-12h in serum. The AUC0-12h (1.61 mcg∙h/mL) of tigecycline in skin blister fluid was approximately 26% lower than the AUC0-12h in the serum of 10 healthy subjects. In a single-dose study, tigecycline 100 mg was administered to subjects prior to undergoing elective surgery or medical procedure for tissue extraction. Concentrations at 4 hours after tigecycline administration were higher in gallbladder (38-fold, n=6), lung (3.7-fold, n=5), and colon (2.3-fold, n=6), and lower in synovial fluid (0.58-fold, n=5), and bone (0.35-fold, n=6) relative to serum. The concentration of tigecycline in these tissues after multiple doses has not been studied.
- Metabolism:
- Tigecycline is not extensively metabolized. No known relevant CYP450 drug interactions.
- In vitro studies with tigecycline using human liver microsomes, liver slices, and hepatocytes led to the formation of only trace amounts of metabolites. In healthy male volunteers receiving 14C-tigecycline, tigecycline was the primary 14C-labeled material recovered in urine and feces, but a glucuronide, an N-acetyl metabolite, and a tigecycline epimers (each at no more than 10% of the administered dose) were also present.
- Elimination:
- Route of Elimination = 59% (feces) and 33% (urine)
- The recovery of total radioactivity in feces and urine following administration of 14C-tigecycline indicates that 59% of the dose is eliminated by biliary/fecal excretion, and 33% is excreted in urine. Approximately 22% of the total dose is excreted as unchanged tigecycline in urine. Overall, the primary route of elimination for tigecycline is biliary excretion of unchanged tigecycline and its metabolites. Glucuronidation and renal excretion of unchanged tigecycline are secondary routes.
- Special Populations:
- Hepatic Impairment: In a study comparing 10 patients with mild hepatic impairment (Child Pugh A), 10 patients with moderate hepatic impairment (Child Pugh B), and 5 patients with severe hepatic impairment (Child Pugh C) to 23 age and weight matched healthy control subjects, the single-dose pharmacokinetic disposition of tigecycline was not altered in patients with mild hepatic impairment. However, systemic clearance of tigecycline was reduced by 25% and the half-life of tigecycline was prolonged by 23% in patients with moderate hepatic impairment (Child Pugh B). Systemic clearance of tigecycline was reduced by 55%, and the half-life of tigecycline was prolonged by 43% in patients with severe hepatic impairment (Child Pugh C). Dosage adjustment is necessary in patients with severe hepatic impairment (Child Pugh C).
- Renal Impairment: A single dose study compared 6 subjects with severe renal impairment (creatinine clearance <30 mL/min), 4 end stage renal disease (ESRD) patients receiving tigecycline 2 hours before hemodialysis, 4 ESRD patients receiving tigecycline 1 hour after hemodialysis, and 6 healthy control subjects. The pharmacokinetic profile of tigecycline was not significantly altered in any of the renally impaired patient groups, nor was tigecycline removed by hemodialysis. No dosage adjustment is necessary in patients with renal impairment or in patients undergoing hemodialysis.
- Geriatric Patients: No significant differences in pharmacokinetics were observed between healthy elderly subjects (n=15, age 65-75; n=13, age >75) and younger subjects (n=18) receiving a single 100-mg dose of tigecycline. Therefore, no dosage adjustment is necessary based on age.
- Pediatric Patients:
- A single-dose safety, tolerability, and pharmacokinetic study of tigecycline in pediatric patients aged 8-16 years who recently recovered from infections was conducted. The doses administered were 0.5, 1, or 2 mg/kg. The study showed that for children aged 12-16 years (n = 16) a dosage of 50 mg twice daily would likely result in exposures comparable to those observed in adults with the approved dosing regimen. Large variability observed in children aged 8 to 11 years of age (n = 8) required additional study to determine the appropriate dosage.
- A subsequent tigecycline dose-finding study was conducted in 8-11 year old patients with cIAI, cSSSI, or CABP. The doses of tigecycline studied were 0.75 mg/kg (n = 17), 1 mg/kg (n = 21), and 1.25 mg/kg (n=20). This study showed that for children aged 8-11 years, a 1.2 mg/kg dose would likely result in exposures comparable to those observed in adults resulting with the approved dosing regimen.
- Gender: In a pooled analysis of 38 women and 298 men participating in clinical pharmacology studies, there was no significant difference in the mean (±SD) tigecycline clearance between women (20.7±6.5 L/h) and men (22.8±8.7 L/h). Therefore, no dosage adjustment is necessary based on gender.
- Race: In a pooled analysis of 73 Asian subjects, 53 Black subjects, 15 Hispanic subjects, 190 White subjects, and 3 subjects classified as "other" participating in clinical pharmacology studies, there was no significant difference in the mean (±SD) tigecycline clearance among the Asian subjects (28.8±8.8 L/h), Black subjects (23.0±7.8 L/h), Hispanic subjects (24.3±6.5 L/h), White subjects (22.1±8.9 L/h), and "other" subjects (25.0±4.8 L/h). Therefore, no dosage adjustment is necessary based on race.
- Drug Interactions:
- Tigecycline (100 mg followed by 50 mg every 12 hours) and digoxin (0.5 mg followed by 0.25 mg, orally, every 24 hours) were co-administered to healthy subjects in a drug interaction study. Tigecycline slightly decreased the Cmax of digoxin by 13%, but did not affect the AUC or clearance of digoxin. This small change in Cmax did not affect the steady-state pharmacodynamic effects of digoxin as measured by changes in ECG intervals. In addition, digoxin did not affect the pharmacokinetic profile of tigecycline. Therefore, no dosage adjustment of either drug is necessary when tigecycline is administered with digoxin.
- Concomitant administration of tigecycline (100 mg followed by 50 mg every 12 hours) and warfarin (25 mg single-dose) to healthy subjects resulted in a decrease in clearance of R-warfarin and S-warfarin by 40% and 23%, an increase in Cmax by 38% and 43% and an increase in AUC by 68% and 29%, respectively. Tigecycline did not significantly alter the effects of warfarin on INR. In addition, warfarin did not affect the pharmacokinetic profile of tigecycline. However, prothrombin time or other suitable anticoagulation test should be monitored if tigecycline is administered with warfarin.
- In vitro studies in human liver microsomes indicate that tigecycline does not inhibit metabolism mediated by any of the following 6 cytochrome P450 (CYP) isoforms: 1A2, 2C8, 2C9, 2C19, 2D6, and 3A4. Therefore, tigecycline is not expected to alter the metabolism of drugs metabolized by these enzymes. In addition, because tigecycline is not extensively metabolized, clearance of tigecycline is not expected to be affected by drugs that inhibit or induce the activity of these CYP450 isoforms.
- Patients should be counseled that antibacterial drugs, including tigecycline, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold).
- When tigecycline is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by tigecycline or other antibacterial drugs in the future.
- Patients should be advised that diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued. Sometimes after starting treatment, patients can develop watery and bloody stools (with o without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
- Advise patients of the risk of fetal harm during pregnancy
Indications
Dosing (Adult)
General Dosing & Administration Notes: Give IV over 30-60 minutes. May administer IV through a dedicated line or through a Y-site. Flush line with 0.9% NaCl injection, D5 injection, or lactated Ringer's injection before and after infusion of therapy if the same IV line is used for sequential infusion of several drugs
Dosing (Pediatric)
Black Box Warnings
Warnings
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

