Ramipril (Altace): Drug Monograph
|
|---|
- Hypertension alone or in combination with thiazide diuretics
- Reduce the risk of myocardial infarction, stroke, or death from cardiovascular causes in patients ≥ 55 years of age at high risk of developing a major CV event due to history of coronary artery disease, stroke, peripheral vascular disease, or diabetes with at least 1 other CV risk factor
- Heart failure within the 1st few days after sustaining acute MI
- General Notes:
- Swallow capsules whole.
- May sprinkle contents on a small amount (about 4 oz) of applesauce or mix in 4 ox of water or apple juice.
- Consume mixture in its entirety.
- May pre-prepare and store described mixture for up to 24 hours at room temperature or up to 48 hours under refrigeration.
- Congestive heart failure post-myocardial infarction:
- 2.5 mg by mouth twice daily initially (or) may switch to 1.25 mg twice daily if hypotensive at this dose
- If tolerated, increase to 5 mg twice daily at 3-week intervals after 1 week of initial dose
- After initial dose, observe for at least 2 hours and until blood pressure has stabilized for at least an additional hour
- Hypertension:
- 2.5 mg by mouth daily initially
- Adjust dosage according to blood pressure response after 2-4 weeks of treatment
- Maintenance: 2.5 mg-20 mg daily as a single dose or in 2 equally divided doses.
- May add diuretic if blood pressure is not controlled
- Reduction in the risk of myocardial infarction, stroke, or death from cardiovascular causes, ≥55 years:
- 2.5 mg by mouth once daily for 1 week
- Increase to 5 mg once daily for 3 weeks, and increased as tolerated
- Maintenance: 10 mg once daily. May be given as a divided dose if hypertensive or recently post-MI
- CrCl ≤ 40 mL/min:
- Administer 25% of the initial or recommended dose
- HTN: 1.25 daily initially. May increase dosage until blood pressure is controlled. Maximum: 5 mg/day
- CHF post MI: 1.25 mg daily initially. May increase to 1.25 mg twice daily. Maximum: 2.5 mg twice daily
- Fetal Toxicity - when pregnancy is detected, discontinue ramipril as soon as possible. Can cause injury and death to the developing fetus.
- Angioedema related to previous treatment with an ACE inhibitor, or a history of hereditary or idiopathic angioedema
- Do not co-administer aliskiren in patients with diabetes
- Angioedema - increased risk in patients with a prior history
- Hypotension and hyperkalemia
- Renal impairment - monitor renal function during therapy
- Avoid concomitant use of an ACE inhibitor and angiotensin blocker
- Rare cholestatic jaundice and hepatic failure
- Rare neutropenia and agranulocytosis
- Neutropenia and agranulocytosis - rare incidences of mild reductions in red blood cell count and hemoglobin count, blood cell or platelet counts.
- Dual blockade of the renin-angiotensin system - increased risk of hypotension, hyperkalemia, and changes in renal function when used with ACE inhibitors or aliskiren
- Limited data on human overdosage are available. The most likely clinical manifestations would be symptoms attributable to hypotension.
- No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) or hemodialysis that might accelerate elimination of ramipril and its metabolites.
- Reasonable to treat overdose by infusion of normal saline solution because the hypotensive effect of ramipril is achieved through vasodilation and effective hypovolemia.
- Diuretics - possibility of excessive hypotension
- RAS inhibitors - in general, avoid combined use. Do not co-administer aliskiren with ramipril in patients with diabetes.
- Lithium - use with caution
- Gold - nitritoid reactions have been reported
- NSAIDs - may lead to increased risk of renal impairment and loss of antihypertensive effect
- mTOR inhibitor - may increase angioedema risk
- Pregnancy: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: Not recommended for use in nursing mothers.
- Renal Impairment: Starting dose should be adjusted downward in patients with moderate-to-severe impairment.
- Hepatic Impairment: None
- Pediatric Patients: Safety and effectiveness have not been established. Neonates with a history of in utero exposure to ramipril: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
- Geriatric Patients: No overall differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
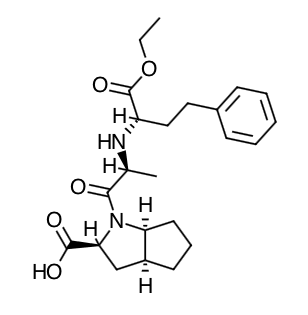
- Scientific Name: (2S,3aS,6aS)-1[(S)-N-[(S)-1-Carboxy-3-phenylpropyl] alanyl] octahydrocyclopenta [b]pyrrole-2-carboxylic acid, 1-ethyl ester
- Empirical Formula: C23H32N2O5
- Molecular Weight: 416.5
- Ramipril and ramiprilat inhibit ACE in human subjects and animals. Angiotensin converting enzyme is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with ramipril alone for up to 56 weeks, approximately 4% of patients during the trial had abnormally high serum potassium and an increase from baseline greater than 0.75 mEq/L, and none of the patients had an abnormally low potassium and a decrease from baseline greater than 0.75 mEq/L. In the same study, approximately 2% of patients treated with ramipril and hydrochlorothiazide for up to 56 weeks had abnormally high potassium values and an increase from baseline of 0.75 mEq/L or greater; and approximately 2% had abnormally low values and decreases from baseline of 0.75 mEq/L or greater. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
- The effect of ramipril on hypertension appears to result at least in part from inhibition of both tissue and circulating ACE activity, thereby reducing angiotensin II formation in tissue and plasma.
- Angiotensin converting enzyme is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasopressor peptide, play a role in the therapeutic effects of ramipril remains to be elucidated.
- While the mechanism through which ramipril lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, it has an antihypertensive effect even in patients with low-renin hypertension. Although ramipril was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a blood pressure lowering response to monotherapy, albeit a smaller average response, than non-Black patients.
- Single doses of ramipril of 2.5 mg-20 mg produce approximately 60%-80% inhibition of ACE activity 4 hours after dosing with approximately 40%-60% inhibition after 24 hours. Multiple oral doses of ramipril of 2.0 mg or more cause plasma ACE activity to fall by more than 90% 4 hours after dosing, with over 80% inhibition of ACE activity remaining 24 hours after dosing. The more prolonged effect of even small multiple doses presumably reflects saturation of ACE binding sites by ramiprilat and relatively slow release from those sites.
- Absorption: Following oral administration of ramipril, peak plasma concentrations (Cmax) of ramipril are reached within 1 hour. The extent of absorption is at least 50%-60%, and is not significantly influenced by the presence of food in the gastrointestinal tract, although the rate of absorption is reduced. In a trial in which subjects received ramipril capsules or the contents of identical capsules dissolved in water, dissolved in apple juice, or suspended in applesauce, serum ramiprilat levels were essentially unrelated to the use or non-use of the concomitant liquid or food.
- Distribution: Cleavage of the ester group (primarily in the liver) converts ramipril to its active diacid metabolite, ramiprilat. Peak plasma concentrations of ramiprilat are reached 2-4 hours after drug intake. The serum protein binding of ramipril is about 73% and that of ramiprilat about 56%; in vitro, these percentages are independent of concentration over the range of 0.01 µg/mL-10 µg/mL.
- Metabolism: Ramipril is almost completely metabolized to ramiprilat, which has about 6 times the ACE inhibitory activity of ramipril, and to the diketopiperazine ester, the diketopiperazine acid, and the glucuronides of ramipril and ramiprilat, all of which are inactive. Plasma concentrations of ramipril and ramiprilat increase with increased dose, but are not strictly dose-proportional. The 24-hour AUC for ramiprilat, however, is dose-proportional over the 2.5 mg-20 mg dose range. The absolute bioavailabilities of ramipril and ramiprilat were 28% and 44%, respectively, when 5 mg of oral ramipril was compared with the same dose of ramipril given intravenously. After once-daily dosing, steady-state plasma concentrations of ramiprilat are reached by the fourth dose. Steady-state concentrations of ramiprilat are somewhat higher than those seen after the first dose of ramipril, especially at low doses (2.5 mg), but the difference is clinically insignificant. Plasma concentrations of ramiprilat decline in a triphasic manner (initial rapid decline, apparent elimination phase, terminal elimination phase). The initial rapid decline, which represents distribution of the drug into a large peripheral compartment and subsequent binding to both plasma and tissue ACE, has a half-life of 2-4 hours. Because of its potent binding to ACE and slow dissociation from the enzyme, ramiprilat shows two elimination phases. The apparent elimination phase corresponds to the clearance of free ramiprilat and has a half-life of 9-18 hours. The terminal elimination phase has a prolonged half-life (>50 hours) and probably represents the binding/dissociation kinetics of the ramiprilat/ACE complex. It does not contribute to the accumulation of the drug. After multiple daily doses of ramipril 5 mg-10 mg, the half-life of ramiprilat concentrations within the therapeutic range was 13-17 hours. In patients with creatinine clearance <40 mL/min/1.73 m2, peak levels of ramiprilat are approximately doubled, and trough levels may be as much as quintupled. In multiple-dose regimens, the total exposure to ramiprilat (AUC) in these patients is 3-4 times as large as it is in patients with normal renal function who receive similar doses. In patients with impaired liver function, the metabolism of ramipril to ramiprilat appears to be slowed, possibly because of diminished activity of hepatic esterases, and plasma ramipril levels in these patients are increased about 3-fold. Peak concentrations of ramiprilat in these patients, however, are not different from those seen in subjects with normal hepatic function, and the effect of a given dose on plasma ACE activity does not vary with hepatic function.
- Elimination: After oral administration of ramipril, about 60% of the parent drug and its metabolites are eliminated in the urine, and about 40% is found in the feces. Drug recovered in the feces may represent both biliary excretion of metabolites and/or unabsorbed drug, however the proportion of a dose eliminated by the bile has not been determined. Less than 2% of the administered dose is recovered in urine as unchanged ramipril. The urinary excretion of ramipril, ramiprilat, and their metabolites is reduced in patients with impaired renal function. Compared to normal subjects, patients with creatinine clearance <40 mL/min/1.73 m2 had higher peak and trough ramiprilat levels and slightly longer times to peak concentrations.
- Angioedema, including laryngeal edema, can occur rarely with treatment with ACE inhibitors, especially following the first dose. Advise patients to report immediately any signs or symptoms suggesting angioedema (swelling of face, eyes, lips, or tongue, or difficulty in breathing) and to take no more drug until they have consulted with the prescribing physician.
- Advise patients to report promptly any indication of infection (e.g., sore throat, fever), which could be a sign of neutropenia.
- Inform patients that light-headedness can occur, especially during the first days of therapy, and it should be reported. Advise patients to discontinue ramipril if syncope (fainting) occurs, and to follow up with their health care providers.
- Inform patients that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting while taking ramipril can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
- Female patients of childbearing age should be told about the consequences of exposure to ramipril during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
- Advise patients not to use salt substitutes containing potassium without consulting their physician.
Indications
Dosing (Adult)
Renal Dosing
Black Box Warnings
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|