Vigabatrin (Sabril): Drug Monograph
|
|---|
- Adjunctive therapy for adults and pediatric patients ≥10 years of age with refractory complex partial seizures who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss
- Monotherapy for pediatric patients (1 month-2 years of age) with infantile spasms for whom the potential benefits outweigh the potential risk of vision loss (Solution)
- Refractory complex partial seizures, >16 years:
- 1000 mg/day (500 mg twice daily)
- May increase total daily dose in 500 mg increments at weekly intervals to a recommended dose of 3000 mg/day (1500 mg twice daily)
- Infantile spasms:
- 50 mg/kg/day given in 2 divided doses
- Subsequent dosing can be titrated by 25-50 mg/kg/day increments every 3 days
- Maximum: 150 mg/kg/day in 2 divided doses
- Refractory complex partial seizures, 10-16 years:
- 25-60 kg: 500 mg/day (250 mg twice daily)
- May increase weekly to a total maintenance dose of 2000 mg/day (1000 mg twice daily)
- >60 kg: Dose according to adult recommendations
- Mild, ClCr >50-80 mL/min:
- Decrease dose by 25%
- Moderate, ClCr>30-50 mL/min:
- Decrease dose by 50%
- Severe, ClCr >10-30 mL/min:
- Decrease dose by 75%
-
Vigabatrin causes permanent bilateral concentric visual field constriction. Because assessing vision may be difficult in infants and children, the frequency and extent of vision loss is poorly characterized in these patients. For this reason, the risk described below is primarily based on the adult experience.
-
Based upon adult studies, 30 percent or more of patients can be affected, ranging in severity from mild to severe, including tunnel vision to within 10 degrees of visual fixation, and can result in disability. In some cases, vigabatrin also can damage the central retina and may decrease visual acuity.
-
The onset of vision loss from vigabatrin is unpredictable, and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years.
-
Symptoms of vision loss from vigabatrin are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function.
-
The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss.
-
Unless a patient is formally exempted from periodic ophthalmologic assessment as documented in the SHARE program, vision should be assessed to the extent possible at baseline (no later than 4 weeks after starting vigabatrin) and at least every 3 months during therapy. Vision assessment is also required about 3 to 6 months after the discontinuation of vigabatrin therapy.
-
Once detected, vision loss due to vigabatrin is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss.
-
Drug discontinuation should be considered, balancing benefit and risk, if visual loss is documented.
-
It is possible that vision loss can worsen despite discontinuation of vigabatrin.
-
Because of the risk of visual loss, vigabatrin should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2-4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for vigabatrin should be periodically reassessed.
-
Vigabatrin should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks. The interaction of other types of irreversible vision damage with vision damage from vigabatrin has not been well-characterized, but is likely adverse.
-
Vigabatrin should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks.
-
The possibility that vision loss from vigabatrin may be more common, more severe or have more severe functional consequences in infants and children than in adults cannot be excluded.
-
The lowest dose and shortest exposure to vigabatrin consistent with clinical objectives should be used.
-
Because of the risk of permanent vision loss, vigabatrin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the SHARE Program
- Permanent vision loss. Baseline and periodic vision assessment is required.
- Abnormal MRI signal changes reported in some infants with IS receiving vigabatrin.
- Suicidal behavior and ideation: Antiepileptic drugs increase the risk of these thoughts and behavior.
- Withdrawal of AEDs: dose should be tapered gradually to avoid withdrawal seizures.
- Anemia
- Somnolence and fatigue
- Peripheral Neuropathy
- Weight Gain
- Edema
- Refractory complex partial seizures: Adults: permanent vision loss, fatigue, somnolence, nystagmus, tremor, vision blurred, memory impairment, weight gain, arthralgia, abnormal coordination, and confusional state.
- Pediatrics 10 to 16 years of age: increased weight, upper respiratory tract infection, tremor, fatigue, aggression and diplopia.
- Infantile Spasms: somnolence, bronchitis, ear infection, and otitis media acute.
-
Coma, unconsciousness, and/or drowsiness common symptoms. Other symptoms may include: vertigo, psychosis, apnea or respiratory depression, bradycardia, agitation, irritability, confusion, headache, hypotension, abnormal behavior, increased seizure activity, status epilepticus, and speech disorder.
-
Standard measures to remove unabsorbed drug should be used, including elimination by emesis or gastric lavage.
- Pregnancy: Pregnancy Category C.
- Labor and Delivery: None
- Nursing Mothers: Vigabatrin is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug.
- Renal Impairment: Dose adjustment recommended.
- Hepatic Impairment: None
- Pediatric Patients: The safety and effectiveness of vigabatrin as adjunctive treatment of refractory complex partial seizures in pediatric patients aged 10 to 16 years of age have been established, but not with patients under 10 years of age. The safety and effectiveness as mono-therapy for pediatric patients with infantile spasms (1 month to 2 years of age) have been established.
- Geriatric Patients: Care should be taken in dose selection, due to the possibility of impaired renal function in the elderly.
-
Vigabatrin is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug.
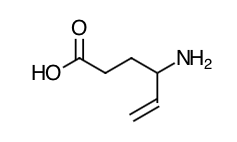
- Scientific Name: Racemate consisting of two enantiomers: (±)4-amino-5-hexenoic acid.
- Empirical Formula: C6H11NO2
- Molecular Weight: 129.16
- The precise mechanism of vigabatrin's anti-seizure effect is unknown, but it is believed to be the result of its action as an irreversible inhibitor of ?-aminobutyric acid transaminase (GABA-T), the enzyme responsible for the metabolism of the inhibitory neurotransmitter GABA. This action results in increased levels of GABA in the central nervous system. No direct correlation between plasma concentration and efficacy has been established. The duration of drug effect is presumed to be dependent on the rate of enzyme re-synthesis rather than on the rate of elimination of the drug from the systemic circulation.
- Effects on Electrocardiogram: There is no indication of a QT/QTc prolonging effect of vigabatrin in single doses up to 6.0 g. In a randomized, placebo-controlled, crossover study, 58 healthy subjects were administered a single oral dose of vigabatrin (3 g and 6 g) and placebo. Peak concentrations for 6.0 g vigabatrin were approximately 2-fold higher than the peak concentrations following the 3.0 g single oral dose.
-
Vigabatrin displayed linear pharmacokinetics after administration of single doses ranging from 0.5 g to 4 g, and after administration of repeated doses of 0.5 g and 2.0 g twice daily. Bioequivalence has been established between the oral solution and tablet formulations. The following PK information (Tmax, half-life, and clearance) of vigabatrin was obtained from stand-alone PK studies and population PK analyses.
-
Absorption: Following oral administration, vigabatrin is essentially completely absorbed. The time to maximum concentration (Tmax) is approximately 1 hour for children (10 years-16 years) and adults, and approximately 2.5 hours for infants (5 months-2 years). There was little accumulation with multiple dosing in adult and pediatric patients. A food effect study involving administration of vigabatrin to healthy volunteers under fasting and fed conditions indicated that the Cmax was decreased by 33%, Tmax was increased to 2 hours, and AUC was unchanged under fed conditions.
-
Distribution: Vigabatrin does not bind to plasma proteins. Vigabatrin is widely distributed throughout the body; mean steady-state volume of distribution is 1.1 L/kg (CV = 20%).
-
Metabolism: Vigabatrin is not significantly metabolized.
-
Elimination: Vigabatrin is eliminated primarily through renal excretion. The terminal half-life of vigabatrin is about 5.7 hours for infants (5 months - 2 years), 9.5 hours for children (10 years - 16 years), and 10.5 hours for adults. Following administration of [14]C-vigabatrin to healthy male volunteers, about 95% of total radioactivity was recovered in the urine over 72 hours with the parent drug representing about 80% of this. Vigabatrin induces CYP2C9, but does not induce other hepatic cytochrome P450 enzyme systems.
-
Special Populations
-
Geriatric: The renal clearance of vigabatrin in healthy elderly patients (≥65 years of age) was 36% less than those in healthy younger patients. This finding is confirmed by an analysis of data from a controlled clinical trial.
-
Pediatric: The clearance of vigabatrin is 2.4 L/hr for infants (5 months-2 years), 5.8 L/hr for children (10 years-16 years) and 7 L/hr for adults.
-
Gender: No gender differences were observed for the pharmacokinetic parameters of vigabatrin in patients.
-
Race: No specific study was conducted to investigate the effects of race on vigabatrin pharmacokinetics. A cross study comparison between 23 Caucasian and 7 Japanese patients who received 1, 2, and 4 g of vigabatrin indicated that the AUC, C max, and half-life were similar for the two populations. However, the mean renal clearance of Caucasians (5.2 L/hr) was about 25% higher than the Japanese (4.0 L/hr). Inter-subject variability in renal clearance was 20% in Caucasians and was 30% in Japanese.
-
Renal Impairment: Mean AUC increased by 30% and the terminal half-life increased by 55% (8.1 hr vs 12.5 hr) in adult patients with mild renal impairment (ClCr from >50-80 mL/min) in comparison to normal subjects.
-
Mean AUC increased by two-fold and the terminal half-life increased by two-fold in adult patients with moderate renal impairment (ClCr from >30-50 mL/min) in comparison to normal subjects.
-
Mean AUC increased by 4.5-fold and the terminal half-life increased by 3.5-fold in adult patients with severe renal impairment (ClCr from >10-30 mL/min) in comparison to normal subjects.
-
Adult patients with renal impairment: Dosage adjustment, including starting at a lower dose, is recommended for adult patients with any degree of renal impairment.
-
Infants with renal impairment: Information about how to adjust the dose in infants with renal impairment is unavailable.
-
Pediatric patients 10 years and older with renal impairment: Although information is unavailable on the effects of renal impairment on vigabatrin clearance in pediatric patients 10 years and older, dosing can be calculated based upon adult data and an established formula
-
Hepatic Impairment: Vigabatrin is not significantly metabolized. The pharmacokinetics of vigabatrin in patients with impaired liver function has not been studied.
- Patients should be informed of the risk of permanent vision loss, particularly loss of peripheral vision, from vigabatrin, and the need for monitoring vision. Monitoring of vision is required at baseline and at least every 3 months while on therapy unless formally exempted as documented by the prescriber. In patients for whom vision testing is not possible, treatment may continue according to clinical judgment with appropriate patient or caregiver counseling and with documentation in the SHARE program of the inability to test vision. Patients should understand that vision testing may be insensitive and may not detect vision loss before it is severe. They should also understand that if vision loss is documented, such loss is irreversible.
- Patients should be informed that if changes in vision are suspected, they should notify their physician immediately.
- Caregivers should be informed of the possibility that infants may develop an abnormal MRI signal of unknown clinical significance.
- Patients should be counseled that vigabatrin may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Behaviors of concern should be reported immediately to health care providers.
- Patients should be instructed to notify their physician if they become pregnant or intend to become pregnant during therapy.
- Patients should notify their physician if they are breastfeeding or intend to breastfeed during therapy.
- Patients should be told not to suddenly discontinue vigabatrin therapy. Withdrawal should be gradual.
- Advise patients not to drive a car or operate other complex machinery until familiar with the effects of therapy on the ability to perform such activities.
Indications
Dosing (Adult)
General Notes: Take with or without food. Either tablet or powder for oral solution can be used for complex partial seizures. Use powder for oral solution for infantile spasms; do not use tablets
Dosing (Pediatric)
Renal Dosing (≥10 years of age)
Black Box Warnings
Warnings
Adverse Reactions
Overdose
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

