Vardenafil (Levitra, Staxyn): Drug Monograph
|
|---|
- Erectile Dysfunction (General Info):
- 10 mg as needed, 60 minutes prior to sexual activity
- Increase to 20 mg or decrease to 5 mg based on efficacy/tolerability
- Note: Maximum: 1 tablet/day
- Elderly patients (≥65 years of age):
- Limit dose to 5 mg
- Patients on stable alpha-blocker therapy:
- Limit dose to 5 mg
- Consider a time interval between dosing
- For patients taking potent or moderate inhibitors of CYP3A4, dose adjustment is as follows:
- Ritonavir: no more than 2.5 mg in a 72-hour period
- Indinavir, saquinavir, atazanavir, ketoconazole 400 mg daily, itraconazole 400 mg daily, clarithromycin: no more than 2.5 mg in a 24-hour period
- Ketoconazole 200 mg daily, itraconazole 200 mg daily, erythromycin: no more than 5 mg in a 24-hour period.
- Moderate (Child-Pugh B):
- 5 mg initially
- Maximum: 10 mg
- Severe (Child-Pugh C):
- Avoid use
- Cardiovascular effects - patients should not use Vardenafil if sex is inadvisable due to cardiovascular status.
- Risk of priapism - in the event that an erection lasts more than 4 hours, the patient should seek immediate medical assistance
- Effects on the eye - patients should stop treatment and seek medical attention in the event of sudden loss of vision in one or both eyes, which could be sign of nonarteritic anterior ischemic optic neuropathy (NAION). Use with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a "crowed" optic disc may also be at an increased risk of NAION.
- Sudden hearing loss - patients should stop treatment and seek medical attention in the event of sudden decrease or loss in hearing
- Alpha-blockers - caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (for example, fainting)
- QT prolongation - patients with congenital QT syndrome or taking class IA or III antiarrhythmics should avoid using Vardenafil
- Other erectile dysfunction therapies - the safety and efficacy of vardenafil used in combination with other treatments for ED have not been studied. Use is not recommended.
- Headache
- Flushing
- Nasal congestion
- Dyspepsia
- Sinusitis
- Flu syndrome
- Dizziness
- Increased creatine kinase
- Nausea
- Back pain
- Signs and symptoms may include reversible back pain/myalgia and/or "abnormal vision."
- Standard supportive measures should be taken as required.
- Renal dialysis is not expected to accelerate clearance as vardenafil is highly bound to plasma proteins and not significantly eliminated in the urine.
- Nitrates, alpha-blockers, and antihypertensives - vardenafil can potentiate the hypotensive effects of these drugs
- Potent CYP3A4 inhibitors (i.e., ritonavir, indinavir, ketoconazole) or moderate CYP3A4 inhibitors (i.e., erythromycin) - increases plasma concentrations of vardenafil. Dosage adjustment is necessary.
- Pregnancy: Pregnancy Category B; not indicated for use in women.
- Labor and Delivery: None
- Nursing Mothers: Not indicated for use in women.
- Renal Impairment: Do not use in patients on renal dialysis; no dosage adjustment necessary in patients with ClCr 30-80 mL/min.
- Hepatic Impairment: Do not use in patients with severe impairment (Child-Pugh C); dosage adjustment is necessary in patients with moderate impairment.
- Pediatric Patients: Not indicated for use in pediatric patients; safety and efficacy have not been established in this population.
- Geriatric Patients: No differences in safety and effectiveness noted between elderly (≥65 years of age) and younger patients. However, due to increased vardenafil concentrations in the elderly, a starting dose of 5 mg should be considered in the elderly.
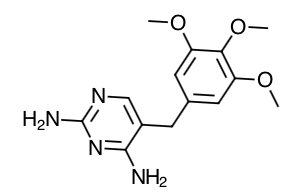
- Scientific Name: piperazine, 1-[[3-(1,4-dihydro-5-methyl- 4-oxo-7-propylimidazo[5,1-f ][1,2,4]triazin-2-yl)-4-ethoxyphenyl]sulfonyl]-4-ethyl-, monohydrochloride
- Empirical Formula: C23H32N6O4S.HCl.3H2O
- Molecular Weight: 579.1
- Penile erection is a hemodynamic process initiated by the relaxation of smooth muscle in the corpus cavernosum and its associated arterioles. During sexual stimulation, nitric oxide is released from nerve endings and endothelial cells in the corpus cavernosum. Nitric oxide activates the enzyme guanylate cyclase resulting in increased synthesis of cyclic guanosine monophosphate (cGMP) in the smooth muscle cells of the corpus cavernosum. The cGMP in turn triggers smooth muscle relaxation, allowing increased blood flow into the penis, resulting in erection. The tissue concentration of cGMP is regulated by both the rates of synthesis and degradation via phosphodiesterases (PDEs). The most abundant PDE in the human corpus cavernosum is the cGMP-specific phosphodiesterase type 5 (PDE5); therefore, the inhibition of PDE5 enhances erectile function by increasing the amount of cGMP. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 has no effect in the absence of sexual stimulation.
- In vitro studies have shown that vardenafil is a selective inhibitor of PDE5. The inhibitory effect of vardenafil is more selective on PDE5 than for other known phosphodiesterases (>15-fold relative to PDE6, >130-fold relative to PDE1, >300-fold relative to PDE11, and >1,000-fold relative to PDE2, 3, 4, 7, 8, 9, and 10).
- Effects on Blood Pressure:
- In a clinical pharmacology study of patients with erectile dysfunction, single doses of vardenafil 20 mg caused a mean maximum decrease in supine blood pressure of 7 mmHg systolic and 8 mmHg diastolic (compared to placebo), accompanied by a mean maximum increase of heart rate of 4 beats per minute. The maximum decrease in blood pressure occurred between 1 and 4 hours after dosing. Following multiple dosing for 31 days, similar blood pressure responses were observed on Day 31 as on Day 1. Vardenafil may add to the blood pressure lowering effects of antihypertensive agents.
- Effects on Blood Pressure and Heart Rate when Combined with Nitrates:
- A study was conducted in which the blood pressure and heart rate response to 0.4 mg nitroglycerin (NTG) sublingually was evaluated in 18 healthy subjects following pretreatment with vardenafil 20 mg at various times before NTG administration. Vardenafil 20 mg caused an additional time-related reduction in blood pressure and increase in heart rate in association with NTG administration. The blood pressure effects were observed when vardenafil 20 mg was dosed 1 or 4 hours before NTG and the heart rate effects were observed when 20 mg was dosed 1, 4, or 8 hours before NTG. Additional blood pressure and heart rate changes were not detected when vardenafil 20 mg was dosed 24 hours before NTG. Because the disease state of patients requiring nitrate therapy is anticipated to increase the likelihood of hypotension, the use of vardenafil by patients on nitrate therapy or on nitric oxide donors is contraindicated.
- Blood Pressure Effects in Patients on Stable Alpha-Blocker Treatment: Three clinical pharmacology studies were conducted in patients with benign prostatic hyperplasia (BPH) on stable-dose alpha-blocker treatment, consisting of alfuzosin, tamsulosin or terazosin.
- Study 1: This study was designed to evaluate the effect of 5 mg vardenafil compared to placebo when administered to BPH patients on chronic alpha-blocker therapy in two separate cohorts: tamsulosin 0.4 mg daily (cohort 1, n=21) and terazosin 5 or 10 mg daily (cohort 2, n=21). The design was a randomized, double blind, cross-over study with four treatments: vardenafil 5 mg or placebo administered simultaneously with the alpha-blocker and vardenafil 5 mg or placebo administered 6 hours after the alpha- blocker. Blood pressure and pulse were evaluated over the 6-hour interval after vardenafil dosing. One patient after simultaneous treatment with 5 mg vardenafil and 10 mg terazosin exhibited symptomatic hypotension with standing blood pressure of 80/60 mmHg occurring one hour after administration and subsequent mild dizziness and moderate lightheadedness lasting for 6 hours. For vardenafil and placebo, five and two patients, respectively, experienced a decrease in standing systolic blood pressure (SBP) of >30 mmHg following simultaneous administration of terazosin. Hypotension was not observed when vardenafil 5 mg and terazosin were administered 6 hours apart. Following simultaneous administration of vardenafil 5 mg and tamsulosin, two patients had a standing SBP of <85 mmHg. A decrease in standing SBP of >30 mmHg was observed in two patients on tamsulosin receiving simultaneous vardenafil and in one patient receiving simultaneous placebo treatment. When tamsulosin and vardenafil 5 mg were separated by 6 hours, two patients had a standing SBP <85 mmHg and one patient had a decrease in SBP of >30 mmHg. There were no severe adverse events related to hypotension reported during the study. There were no cases of syncope.
- Study 2: This study was designed to evaluate the effect of 10 mg vardenafil (stage 1) and 20 mg vardenafil (stage 2) compared to placebo, when administered to a single cohort of BPH patients (n=23) on stable therapy with tamsulosin 0.4 mg or 0.8 mg daily for at least four weeks. The design was a randomized, double blind, two-period cross- over study. Vardenafil or placebo was given simultaneously with tamsulosin. Blood pressure and pulse were evaluated over the 6-hour interval after vardenafil dosing. One patient experienced a decrease from baseline in standing SBP of >30 mmHg following vardenafil 10 mg. There were no other instances of outlier blood pressure values (standing SBP <85 mmHg or decrease from baseline in standing SBP of >30 mmHg). Three patients reported dizziness following vardenafil 20 mg. There were no cases of syncope.
- Study 3: This study was designed to evaluate the effect of single doses of 5 mg vardenafil (stage 1) and 10 mg vardenafil (stage 2) compared to placebo, when administered to a single cohort of BPH patients (n=24) on stable therapy with alfuzosin 10 mg daily for at least four weeks. The design was a randomized, double blind, 3-period cross- over study. Vardenafil or placebo was administered 4 hours after the administration of alfuzosin. Blood pressure and pulse were evaluated over a 10-hour interval after dosing of vardenafil or placebo. One patient experienced decreases from baseline in standing systolic blood pressure >30 mm Hg after administration of vardenafil 5 mg film-coated tablet and vardenafil 10 mg film-coated tablet. No instances of standing systolic blood pressure <85 mm Hg were observed during this study. Four patients, one dosed with placebo, two dosed with vardenafil 5 mg film-coated tablets and one dosed with vardenafil 10 mg film-coated tablets, reported dizziness. Blood pressure effects (standing SBP) in normotensive men on a stable dose of alfuzosin 10 mg following administration of vardenafil 5 mg, vardenafil 10 mg, or placebo separated by 4 hours
- Blood pressure effects in normotensive men after forced titration with alpha-blockers:
- Two randomized, double blind, placebo-controlled clinical pharmacology studies with healthy normotensive volunteers (age range, 45-74 years) were performed after forced titration of the alpha-blocker terazosin to 10 mg daily over 14 days (n=29), and after initiation of tamsulosin 0.4 mg daily for five days (n=24). There were no severe adverse events related to hypotension in either study. Symptoms of hypotension were a cause for withdrawal in 2 subjects receiving terazosin and in 4 subjects receiving tamsulosin. Instances of outlier blood pressure values (defined as standing SBP <85 mmHg and/ or a decrease from baseline of standing SBP >30 mmHg) were observed in 9/24 subjects receiving tamsulosin and 19/29 receiving terazosin. The incidence of subjects with standing SBP <85 mmHg given vardenafil and terazosin to achieve simultaneous Tmax led to early termination of that arm of the study. In most (7/8) of these subjects, instances of standing SBP <85 mmHg were not associated with symptoms. Among subjects treated with terazosin, outlier values were observed more frequently when vardenafil and terazosin were given to achieve simultaneous Tmax than when dosing was administered to separate Tmax by 6 hours. There were 3 cases of dizziness observed with concomitant administration of terazosin and vardenafil. Seven subjects experienced dizziness mainly occurring with simultaneous Tmax administration of tamsulosin. There were no cases of syncope.
- Effects on Cardiac Electrophysiology:
- The effect of 10 mg and 80 mg vardenafil on QT interval was evaluated in a single- dose, double-blind, randomized, placebo- and active-controlled (moxifloxacin 400 mg) crossover study in 59 healthy males (81% White, 12% Black, 7% Hispanic) aged 45-60 years. The QT interval was measured at one-hour post dose because this time point approximates the average time of peak vardenafil concentration. The 80 mg dose of vardenafil (four times the highest recommended dose) was chosen because this dose yields plasma concentrations covering those observed upon co-administration of a low-dose of vardenafil (5 mg) and 600 mg BID of ritonavir. Of the CYP3A4 inhibitors that have been studied, ritonavir causes the most significant drug-drug interaction with vardenafil. No single correction method is known to be more valid than the other. In this study, the mean increase in heart rate associated with a 10 mg dose of vardenafil compared to placebo was 5 beats/minute and with an 80 mg dose the mean increase was 6 beats/minute. Therapeutic and supratherapeutic doses of vardenafil and the active control moxifloxacin produced similar increases in QTc interval. This study, however, was not designed to make direct statistical comparisons between the drug or the dose levels. The clinical impact of these QTc changes is unknown. In a separate post-marketing study of 44 healthy volunteers, single doses of 10 mg vardenafil resulted in a placebo-subtracted mean change from baseline of QTcF (Fridericia correction) of 5 msec (90% CI: 2,8). Single doses of gatifloxacin 400 mg resulted in a placebo-subtracted mean change from baseline QTcF of 4 msec (90% CI: 1,7). When vardenafil 10 mg and gatifloxacin 400 mg were co-administered, the mean QTcF change from baseline was additive when compared to either drug alone and produced a mean QTcF change of 9 msec from baseline (90% CI: 6,11). The clinical impact of these QT changes is unknown.
- Effects on Exercise Treadmill Test in Patients with Coronary Artery Disease (CAD):
- In two independent trials that assessed 10 mg (n=41) and 20 mg (n=39) vardenafil, respectively, vardenafil did not alter the total treadmill exercise time compared to placebo. The patient population included men aged 40-80 years with stable exercise- induced angina documented by at least one of the following: 1) prior history of myocardial infarction (MI), coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or stenting (not within 6 months); 2) positive coronary angiogram showing at least 60% narrowing of the diameter of at least one major coronary artery; or 3) a positive stress echocardiogram or stress nuclear perfusion study. Results of these studies showed that vardenafil did not alter the total treadmill exercise time compared to placebo (10 mg vardenafil vs. placebo: 433±109 and 426±105 seconds, respectively; 20 mg vardenafil vs. placebo: 414±114 and 411±124 seconds, respectively). The total time to angina was not altered by vardenafil when compared to placebo (10 mg vardenafil vs. placebo: 291±123 and 292±110 seconds; 20 mg vardenafil vs. placebo: 354±137 and 347±143 seconds, respectively). The total time to 1 mm or greater ST-segment depression was similar to placebo in both the 10 mg and the 20 mg vardenafil groups (10 mg LEVITRA vs. placebo: 380±108 and 334±108 seconds; 20 mg vardenafil vs. placebo: 364±101 and 366±105 seconds, respectively).
- Effects on Eye:
- Single oral doses of phosphodiesterase inhibitors have demonstrated transient dose- related impairment of color discrimination (blue/green) using the Farnsworth-Munsell 100-hue test and reductions in electroretinogram (ERG) b-wave amplitudes, with peak effects near the time of peak plasma levels. These findings are consistent with the inhibition of PDE6 in rods and cones, which is involved in phototransduction in the retina. The findings were most evident one hour after administration, diminishing but still present 6 hours after administration. In a single dose study in 25 normal males, vardenafil 40 mg, twice the maximum daily recommended dose, did not alter visual acuity, intraocular pressure, fundoscopic and slit lamp findings. In another double blind, placebo controlled clinical trial, at least 15 doses of 20 mg vardenafil were administered over 8 weeks versus placebo to 52 males. Thirty-two (32) males (62%) of the patients completed the trial. Retinal function was measured by ERG and FM-100 test 2, 6 and 24 hours after dosing. The trial was designed to detect changes in retinal function that might occur in more than 10% of patients. Vardenafil did not produce clinically significant ERG or FM-100 effects in healthy men compared to placebo. Two patients on vardenafil in the trial reported episodes of transient cyanopsia (objects appear blue).
- Effects on Sperm Motility/Morphology:
- There was no effect on sperm motility or morphology after single 20 mg oral doses of vardenafil in healthy volunteers.
- The pharmacokinetics of vardenafil are approximately dose proportional over the recommended dose range.
- Absorption: Vardenafil is rapidly absorbed with absolute bioavailability of approximately 15%. Maximum observed plasma concentrations after a single 20 mg dose in healthy volunteers are usually reached between 30 minutes and 2 hours (median 60 minutes) after oral dosing in the fasted state. Two food-effect studies were conducted which showed that high-fat meals caused a reduction in Cmax by 18%-50%.
- Distribution: The mean steady-state volume of distribution (Vss) for vardenafil is 208 L, indicating extensive tissue distribution. Vardenafil and its major circulating metabolite, M1, are highly bound to plasma proteins (about 95% for parent drug and M1). This protein binding is reversible and independent of total drug concentrations. Following a single oral dose of 20 mg vardenafil in healthy volunteers, a mean of 0.00018% of the administered dose was obtained in semen 1.5 hours after dosing.
- Metabolism: Vardenafil is metabolized predominantly by the hepatic enzyme CYP3A4, with contribution from the CYP3A5 and CYP2C isoforms. The major circulating metabolite, M1, results from desethylation at the piperazine moiety of vardenafil. M1 is subject to further metabolism. The plasma concentration of M1 is approximately 26% that of the parent compound. This metabolite shows a phosphodiesterase selectivity profile similar to that of vardenafil and an in vitro inhibitory potency for PDE5 28% of that of vardenafil. Therefore, M1 accounts for approximately 7% of total pharmacologic activity.
- Elimination: The total body clearance of vardenafil is 56 L/h, and the terminal half-life of vardenafil and its primary metabolite (M1) is approximately 4-5 hours. After oral administration, vardenafil is excreted as metabolites predominantly in the feces (approximately 91-95% of administered oral dose) and to a lesser extent in the urine (approximately 2-6% of administered oral dose).
- Specific Populations:
- Pediatrics: Vardenafil is not indicated for use in pediatric patients. Vardenafil trials were not conducted in the pediatric population.
- Geriatric: In a healthy volunteer study of elderly males (≥65 years) and younger males (18-45 years), mean Cmax and AUC were 34% and 52% higher, respectively, in the elderly males.
- Hepatic Impairment: In volunteers with mild hepatic impairment (Child-Pugh A), the Cmax and AUC following a 10 mg vardenafil dose were increased by 22% and 17%, respectively, compared to healthy control subjects. In volunteers with moderate hepatic impairment (Child- Pugh B), the Cmax and AUC following a 10 mg vardenafil dose were increased by 130% and 160%, respectively, compared to healthy control subjects. Vardenafil has not been evaluated in patients with severe (Child-Pugh C) hepatic impairment.
- Renal Impairment: In male volunteers with ClCr= 50-80 mL/min, the pharmacokinetics of vardenafil were similar to those observed in a control group with ClCr >80 mL/min. In male volunteers with ClCr = 30-50 mL/min or ClCr <30 mL/min renal impairment groups, the AUC of vardenafil was 20-30% higher compared to that observed in a control group with ClCr >80 mL/min). Vardenafil pharmacokinetics have not been evaluated in patients requiring renal dialysis.
- Advise patients of the contraindication of vardenafil with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of vardenafil with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
- Inform patients of the potential cardiac risk of sexual activity for patients with preexisting cardiovascular risk factors.
- Inform patients that in some patients concomitant use of PDE5 inhibitors, including vardenafil, with alpha-blockers can lower blood pressure significantly leading to symptomatic hypotension (for example, fainting). Patients prescribed vardenafil who are taking alpha-blockers should be started on the lowest recommended starting dose.
- Patients should be advised of the possible occurrence of symptoms related to postural hypotension and appropriate countermeasures.
- Patients should be advised to contact the prescribing physician if other anti-hypertensive drugs or new medications that may interact with vardenafil are prescribed by another healthcare provider.
- Discuss with patients the appropriate use of vardenafil and its anticipated benefits. It should be explained that sexual stimulation is required for an erection to occur after taking vardenafil. It should be taken approximately 60 minutes before sexual activity.
- Patients should be counseled regarding the dosing of vardenafil especially regarding the maximum daily dose. Patients should be advised to contact their healthcare provider for dose modification if they are not satisfied with the quality of their sexual performance with vardenafil or in the case of an unwanted effect.
- Inform patients that there have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for vardenafil and this class of compounds. In the event that an erection persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
- Advise patients to stop treatment and seek medical attention in the event of sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision, including permanent loss of vision, that has been reported rarely post-marketing in temporal association with the use of all PDE5 inhibitors. It is not possible to determine whether these events were related directly to the use of PDE5 inhibitors or to other factors.
- Inform patients of the increased risk of NAION in individuals who have already experienced NAION in one eye and discuss the increased risk of NAION among the general population in patients with a "crowded" optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitor, including vardenafil, for this uncommon condition.
- Advise patients to stop treatment and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including vardenafil. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors.
- The use of vardenafil offers no protection against sexually transmitted diseases. Counseling of patients about protective measure necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), should be considered.
- Advise patients that the disintegrating tablet (Staxyn) is not interchangeable with the film-coated tablet (Levitra).
Dosing (Adult)
General Notes: May be taken with or without food. (Tablet) Place on tongue to disintegrate; take without liquid and immediately upon removal from blister. Take with or without food. (Disintegrating tablet)
Hepatic Dosing
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|