Valsartan (Diovan): Drug Monograph
|
|---|
- Treatment of hypertension alone or in combination with other antihypertensives
- Treatment of heart failure (NYHA class II-IV)
- Reduction of cardiovascular mortality in clinically stable patients with left ventricular failure/dysfunction following myocardial infarction
- Heart failure:
- 40 mg by mouth twice daily
- May increase to 80 mg and 160 mg twice daily (use highest dose tolerated)
- Maximum: 320 mg/day in divided doses
- Hypertension:
- 80 or 160 mg by mouth once daily
- May increase to a maximum of 320 mg once daily
- May add diuretic if blood pressure is not controlled
- Post-Myocardial Infarction:
- 20 mg by mouth twice daily as early as 12 hours after MI
- May increase to 40 mg twice daily within 7 days, with subsequent titrations to 160 mg twice daily as tolerated
- Maintenance: 160 mg twice daily
- Consider reducing dose is symptomatic hypotension or renal dysfunction occurs
- Hypertension (6-16 years):
- 1.3 mg/kg once daily (up to 40 mg total)
- Adjust dose according to blood pressure response
- Maximum: 2.7 mg/kg (up to 160 mg) once daily
- When pregnancy is detected, discontinue valsartan as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
- Known hypersensitivity to any component
- Do not co-administer with aliskiren in patients with diabetes
- Observe for signs and symptoms of hypotension
- Monitor renal function and potassium in susceptible patients
- Hypertension:
- Headache
- Dizziness
- Viral infection
- Fatigue
- Abdominal pain
- Heart failure:
- Dizziness
- Hypotension
- Diarrhea
- Arthralgia
- Back pain
- Fatigue
- Hyperkalemia
- Post-myocardial infarction:
- Hypotension
- Cough
- Increased blood creatinine
- Limited data are available related to overdosage in humans.
- The most likely manifestations would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. Depressed level of consciousness, circulatory collapse and shock have been reported.
- If symptomatic hypotension should occur supportive treatment should be instituted.
- Not removed from the plasma by hemodialysis.
- Potassium sparing diuretics, potassium supplements or salt substitutes - may lead to increases in serum potassium, and in heart failure patients, increases in serum creatinine
- NSAID - may lead to increased risk of renal impairment and loss of antihypertensive effect
- Dual inhibition of the renin-angiotensin system - increased risk of renal impairment, hypotension, and hyperkalemia
- Lithium - increases in serum lithium concentrations and lithium toxicity
- Pregnancy: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: It is not known whether valsartan is excreted in human milk. Nursing or drug should be discontinued.
- Renal Impairment: Safety and effectiveness in patients with severe impairment (CrCl≤30 mL/min) have not been established. No dose adjustment is required in patients with mild (CrCl 60 to 90 mL/min) or moderate (CrCl 30-60 mL/min) impairment.
- Hepatic Impairment: No dose adjustment is necessary for patients with mild-to-moderate liver disease. No dosing recommendations can be provided for patients with severe liver disease.
- Pediatric Patients: Efficacy and safety data support use in 6-16 year old patients; use is not recommended in patients <6 years old. No data are available I pediatric patients either undergoing dialysis or with a glomerular filtration rate <30 mL/min/1.73 m2.
- Geriatric Patients: No overall difference in the efficacy or safety of valsartan observed with hypertensive patients ≥75 years of age, but greater sensitivity of some older individuals cannot be ruled out
- It is not known whether valsartan is excreted in human milk. Nursing or drug should be discontinued.
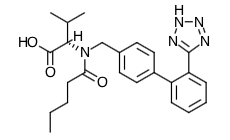
- Scientific Name: N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl) [1,1′-biphenyl]-4-yl]methyl]-L-valine
- Empirical Formula: C24H29N5O3
- Molecular Weight: 435.5
- Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Valsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
- There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostatis. Valsartan has much greater affinity (about 20,000-fold) for the AT1 receptor than for the AT2 receptor. The increased plasma levels of angiotensin II following AT1 receptor blockade with valsartan may stimulate the unblocked AT2 receptor. The primary metabolite of valsartan is essentially inactive with affinity for the AT1 receptor about one-200th that of valsartan itself.
- Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because valsartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Valsartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
- Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of valsartan on blood pressure.
- Valsartan inhibits the pressor effect of angiotensin II infusions. An oral dose of 80 mg inhibits the pressor effect by about 80% at peak with approximately 30% inhibition persisting for 24 hours. No information on the effect of larger doses is available.
- Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin and consequent rise in angiotensin II plasma concentration in hypertensive patients. Minimal decreases in plasma aldosterone were observed after administration of valsartan; very little effect on serum potassium was observed.
- In multiple-dose studies in hypertensive patients with stable renal insufficiency and patients with renovascular hypertension, valsartan had no clinically significant effects on glomerular filtration rate, filtration fraction, creatinine clearance, or renal plasma flow.
- In multiple-dose studies in hypertensive patients, valsartan had no notable effects on total cholesterol, fasting triglycerides, fasting serum glucose, or uric acid.
- Absorption: Valsartan peak plasma concentration is reached 2 to 4 hours after dosing. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. Absolute bioavailability for valsartan is about 25% (range 10% to 35%). The bioavailability of the suspension is 1.6 times greater than with the tablet. With the tablet, food decreases the exposure (as measured by AUC) to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%. AUC and Cmax values of valsartan increase approximately linearly with increasing dose over the clinical dosing range. Valsartan does not accumulate appreciably in plasma following repeated administration.
- Distribution: The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Valsartan is highly bound to serum proteins (95%), mainly serum albumin
- Metabolism: The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism. Following intravenous administration, plasma clearance of valsartan is about 2 L/h and its renal clearance is 0.62 L/h (about 30% of total clearance).
- Elimination: Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites.
- Special Populations
- Pediatric: In a study of pediatric hypertensive patients (n=26, 1 to 16 years of age) given single doses of a suspension of valsartan (mean: 0.9 to 2 mg/kg), the clearance (L/h/kg) of valsartan for children was similar to that of adults receiving the same formulation.
- Geriatric: Exposure (measured by AUC) to valsartan is higher by 70% and the half-life is longer by 35% in the elderly than in the young. No dosage adjustment is necessary.
- Gender: Pharmacokinetics of valsartan does not differ significantly between males and females.
- Heart Failure: The average time to peak concentration and elimination half-life of valsartan in heart failure patients are similar to those observed in healthy volunteers. AUC and Cmax values of valsartan increase linearly and are almost proportional with increasing dose over the clinical dosing range (40 to 160 mg twice a day). The average accumulation factor is about 1.7. The apparent clearance of valsartan following oral administration is approximately 4.5 L/h. Age does not affect the apparent clearance in heart failure patients.
- Renal Insufficiency: There is no apparent correlation between renal function (measured by creatinine clearance) and exposure (measured by AUC) to valsartan in patients with different degrees of renal impairment. Consequently, dose adjustment is not required in patients with mild-to-moderate renal dysfunction. No studies have been performed in patients with severe impairment of renal function (creatinine clearance <10 mL/min). Valsartan is not removed from the plasma by hemodialysis. In the case of severe renal disease, exercise care with dosing of valsartan.
- Hepatic Insufficiency: On average, patients with mild-to-moderate chronic liver disease have twice the exposure (measured by AUC values) to valsartan of healthy volunteers (matched by age, sex, and weight). In general, no dosage adjustment is needed in patients with mild-to-moderate liver disease. Care should be exercised in patients with liver disease.
- Counsel patients about the risk/benefits of therapy and possible adverse effects.
- Female patients of childbearing age should be told about the consequences of exposure to valsartan during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
- Advise women that it is not known if valsartan passes into breast milk. A decision should be made whether to breastfeed or take the medication.
- Advise patients to inform their physician if they have ever had a reaction called angioedema, to another blood pressure medicine. Angioedema causes swelling of the face, lips tongue and/or throat, and may cause difficulty breathing.
- Advise patients to inform their physician of other medicines they are taking including prescription and nonprescription medicines, vitamins and herbal supplements. Especially they should tell their physician if they are taking: other medicines for high blood pressure or a heart problem, water pills (also called "diuretics"), potassium supplements, a salt substitute, nonsteroidal anti-inflammatory drugs (like ibuprofen or naproxen), certain antibiotics (rifamycin group), a drug used to protect against transplant rejection (cyclosporine) or an antiretroviral drug used to treat HIV/AIDS infection (ritonavir), or lithium.
- Advise patients to take the medication exactly as prescribed. Valsartan can be taken with or without food. If a patient misses a dose, they should take it as soon as they remember. If it is close to the next dose, do not take the missed dose. Take the next dose at the regular time.
Indications
Dosing (Adult)
General Notes: Take by mouth with or without food.
Dosing (Pediatric)
General Notes: Use of a suspension is recommended for children who cannot swallow tablets or if calculated dosage does not correspond to available tablet strength. Adjust dose accordingly when switching dosage forms; exposure with suspension is 1.6X greater than with tablet
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





