Valproic Acid (Depakene): Drug Monograph
|
|---|
- Monotherapy and adjunctive therapy for treatment of simple and complex absence seizures, and complex partial seizures that occur either in isolation or in association with other types of seizures
- Adjunctive therapy for multiple seizure types that include absence seizures
- General Dosing & Administration Notes:
- Intended for oral administration, but IV dosage form is available.
- Swallow capsule whole; do not chew.
- To help reduce GI irritation, take with food or slowly build up the dose from an initial low level
- Epilepsy (Simple or Complex Absence Seizures):
- Initial: 15 mg/kg/day by mouth
- Increase weekly by 5-10 mg/kg/day until seizures are controlled or side effects preclude further increases
- Max dose: 60 mg/kg/day
- If total dose exceeds 250 mg/day, give in divided doses
- Refer to PI for initial daily dose guide
- Epilepsy (Partial but Complex):
- Initial: 10-15 mg/kg/day by mouth
- Increase by 5-10 mg/kg/week until optimal response is achieved
- If clinical response has not been achieved, measure plasma levels to determine whether they are in the usually accepted therapeutic range (50-100 mcg/mL)
- Max Dose: 60 mg/kg/day
- If total dose exceeds 250 mg/day, give in divided doses
- Conversion to monotherapy:
- Reduce concomitant antiepilepsy drug by 25% every 2 weeks (starting at initiation or 1-2 weeks after start of therapy)
- Initial: 10-15 mg/kg/day by mouth
- Increase by 5-10 mg/kg/week until optimal response is achieved
- If clinical response has not been achieved, measure plasma levels to determine whether they are in the usually accepted therapeutic range (50-100 mcg/mL)
- Max Dose: 60 mg/kg/day
- Mania (Bipolar Disorder; Note: Act as a Mood Stabilizer):
- Initial (If using Stavor): 750 mg in 2 to 4 divided doses. (If using Extended Release): 25 mg/kg/day by mouth once a day
- Max Dose: 60 mg/kg/day
- Migraine Headache (Prevention):
- Initial: 500 mg by mouth once a day (using extended release formulation) x 7 days and then increase by 500 - 1000 mg daily to response.
- Max Dose: 60 mg/kg/day
-
Epilepsy (Simple or Complex Absence Seizures, ≥ 10 Years of Age):
-
Initial: 15 mg/kg/day by mouth
-
Increase weekly by 5-10 mg/kg/day until seizures are controlled or side effects preclude further increases
-
Max Dose: 60 mg/kg/day
-
If total dose exceeds 250 mg/day, give in divided doses
-
Refer to PI for initial daily dose guide
-
Epilepsy (Partial, but Complex):
-
Initial: 10-15 mg/kg/day
-
Increase by 5-10 mg/kg/week until optimal response is achieved
-
If clinical response has not been achieved, measure plasma levels to determine whether they are in the usually accepted therapeutic range (50-100 mcg/mL)
-
No recommendation regarding safety at doses >60 mg/kg/day
-
If total dose exceeds 250 mg/day, give in divided doses
-
Conversions to Monotherapy, ≥10 years:
-
Reduce concomitant antiepilepsy drug by 25% every 2 weeks (starting at initiation or 1-2 weeks after start of therapy)
-
Initial: 10-15 mg/kg/day
-
Increase by 5-10 mg/kg/week until optimal response is achieved
-
If clinical response has not been achieved, measure plasma levels to determine whether they are in the usually accepted therapeutic range (50-100 mcg/mL)
-
No recommendation regarding safety at doses >60 mg/kg/day
- General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate, usually occurring during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
- Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When valproic acid products are used in this patient group, they should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
- Patients with Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g. Alpers Huttenlocher Syndrome). Valproic Acid is contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, valproic acid should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with valproic acid for the development of acute liver injury with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice.
- Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores following in utero exposure.
- Valproate should only be used to treat pregnant women with epilepsy if other medications have failed to control their symptoms or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate.
- Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated.
- Hepatic disease or significant hepatic dysfunction
- Known hypersensitivity to the drug
- Urea cycle disorders
- Hepatotoxicity; evaluate high risk populations monitor serum liver tests
- Known mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG)
- Suspected POLG-related disorder in children under two years of age
- Birth defects and decreased IQ following in utero exposure; only use to treat pregnant women with epilepsy if other medications are unacceptable; should not be administered to a woman of childbearing potential unless essential
- Pancreatitis; valproic acid should ordinarily be discontinued
- Brain atropy; evaluate for continued use in the presence of suspected or apparent signs of reversible or irreversible cerebral and cerebellar atrophy
- Suicidal behavior or ideation
- Thrombocytopenia; monitor platelet counts and coagulation tests
- Hyperammonemia and hyperammonemic encephalopathy; measure ammonia level if unexplained lethargy and vomiting or changes in mental status
- Hypothermia
- Multi-organ hypersensitivity reaction; discontinue valproic acid
- Somnolence in the elderly. Increase dosage slowly and with regular monitoring.
- Abdominal pain
- Alopecia
- Amblyopia/blurred vision
- Amnesia
- Anorexia
- Asthenia
- Ataxia
- Bronchitis
- Constipation
- Depression
- Diarrhea
- Diplopia
- Dizziness
- Dyspepsia
- Dyspnea
- Ecchymosis
- Emotional lability
- Fever
- Flu syndrome
- Headache
- Increased appetite
- Infection
- Insomnia
- Nausea
- Nervousness
- Nystagmus
- Peripheral edema
- Pharyngitis
- Rhinitis
- Somnolence
- Thinking abnormal
- Thrombocytopenia
- Tinnitus
- Tremor
- Vomiting
- Weight gain, weight loss
- Safety and tolerability of valproate in pediatric patients comparable to adults.
- Somnolence, heart block, and deep coma. Fatalities have been reported.
- The fraction of drug not bound to protein is high and hemodialysis or tandem hemodialysis plus hemoperfusion may result in significant removal of drug.
- The benefit of gastric lavage or emesis will vary with the time since ingestion.
- General supportive measure should be applied with particular attention to the maintenance of adequate urinary output.
- Naloxone has been reported to reverse the CNS depressant effects of overdosage; use with caution.
- Hepatic enzyme-inducing drugs (e.g., phenytoin, carbamazepine, phenobarbital, primidone, rifampin) can increase valproate clearance, while enzyme inhibitors (e.g., felbamate) can decrease valproate clearance. Therefore increased monitoring of valproate and concomitant drug concentrations and dosage adjustment are indicated whenever enzyme-inducing or inhibiting drugs are introduced or withdrawn
- Aspirin, carbapenem antibiotics: Monitoring of valproate concentrations is recommended
- Co-administration of valproate can affect the pharmacokinetics of other drugs (e.g. diazepam, ethosuximide, lamotrigine, phenytoin) by inhibiting their metabolism or protein binding displacement
- Dosage adjustment of amitryptyline/nortryptyline, warfarin, and zidovudine may be necessary if used concomitantly with Depakene
- Topiramate: Hyperammonemia and encephalopathy
- Pregnancy: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: Valproic acid is excreted in human milk.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Children under the age of two years are at considerably higher risk of fatal hepatotoxicity. Above the age of 2 years, the incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
- Geriatric Patients: Reduce starting dose; increase dosage more slowly; monitor fluid and nutritional intake, and somnolence.
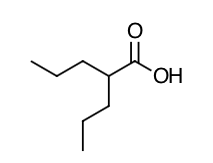
- Scientific Name: 2-propylpentanoic acid
- Empirical Formula: C8H16O2
- Molecular Weight: 144
- Valproic acid dissociates to the valproate ion in the gastrointestinal tract. The mechanisms by which valproate exerts its antiepileptic effects have not been established. It has been suggested that its activity in epilepsy is related to increased brain concentrations of gamma-aminobutyric acid (GABA).
- The relationship between plasma concentration and clinical response is not well documented. One contributing factor is the nonlinear, concentration dependent protein binding of valproate, which affects the clearance of the drug. Thus, monitoring of total serum valproate cannot provide a reliable index of the bioactive valproate species.
- For example, because the plasma protein binding of valproate is concentration dependent, the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Higher than expected free fractions occur in the elderly, in hyperlipidemic patients, and in patients with hepatic and renal diseases.
- Epilepsy: The therapeutic range is commonly considered to be 50 to 100 mcg/mL of total valproate, although some patients may be controlled with lower or higher plasma concentrations.
- Absorption: Equivalent oral doses of Depakote (divalproex sodium) products and Depakene (valproic acid) capsules deliver equivalent quantities of valproate ion systemically. Although the rate of valproate ion absorption may vary with the formulation administered (liquid, solid, or sprinkle), conditions of use (e.g., fasting or postprandial) and the method of administration (e.g., whether the contents of the capsule are sprinkled on food or the capsule is taken intact), these differences should be of minor clinical importance under the steady state conditions achieved in chronic use in the treatment of epilepsy. However, it is possible that differences among the various valproate products in Tmax and Cmax could be important upon initiation of treatment. For example, in single dose studies, the effect of feeding had a grater influence on the rate of absorption of the Depakote tablet (increase in Tmax from 4 to 8 hours) than on the absorption of the Depakote sprinkle capsules (increase in Tmax from 3.3 to 4.8 hours). While the absorption rate from the G.I. tract and fluctuation in valproate plasma concentrations vary with dosing regimen and formulation, the efficacy of valproate as an anticonvulsant in chronic use is unlikely to be affected. Experience employing dosing regimens from once-a-day to four-times-a-day, as well as studies in primate epilepsy models involving constant rate infusion, indicate that total daily systemic bioavailability (extent of absorption) is the primary determinant of seizure control and that differences in the ratios of plasma peak to trough concentrations between valproate formulations are inconsequential from a practical clinical standpoint. Co-administration of oral valproate products with food and substitution among the various Depakote and Depakene formulations should cause no clinical problems in the management of patients with epilepsy. Nonetheless, any changes in dosage administration, or the addition or discontinuance of concomitant drugs should ordinarily be accompanied by close monitoring of clinical status and valproate plasma concentrations.
- Distribution: Protein Binding: The plasma protein binding of valproate is concentration dependent and the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Protein binding of valproate is reduced in the elderly, in patients with chronic hepatic diseases, in patients with renal impairment, and in the presence of other drugs (e.g., aspirin). Conversely, valproate may displace certain protein-bound drugs (e.g., phenytoin, carbamazepine, warfarin, and tolbutamide). CNS Distribution: Valproate concentrations in cerebrospinal fluid (CSF) approximate unbound concentrations in plasma (about 10% of total concentration).
- Metabolism: Valproate is metabolized almost entirely by the liver. In adult patients on mono-therapy, 30-50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial β-oxidation is the major metabolic pathway, typically accounting for over 40% of the dose. Usually, less than 15-20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine. The relationship between dose and total valproate concentration is nonlinear; concentration does not increase proportionally with the dose, but rather, increases to a lesser extent due to saturable plasma protein binding. The kinetics of unbound drug are linear.
- Elimination: Mean plasma clearance and volume of distribution for total valproate are 0.56 L/hr.1.73 m2 and 11 L/1.73 m2, respectively. Mean plasma clearance and volume of distribution for free valproate are 4.6 L/hr/1.73 m2 and 92 L/1.73 m2. Mean terminal half-life for valproate mono-therapy ranged from 9 to 16 hours following oral dosing regimens of 250 to 1000 mg. The estimates cited apply primarily to patients who are not taking drugs that affect hepatic metabolizing enzyme systems. For example patients taking enzyme-inducing antiepileptic drugs (carbamazepine, phenytoin, and phenobarbital) will clear valproate more rapidly. Because of these changes in valproate clearance, monitoring of antiepileptic concentrations should be intensified whenever concomitant antiepileptics are introduced or withdrawn.
- Special Populations
- Neonates: Children within the first two months of life have a markedly decreased ability to eliminate valproate compared to older children and adults. This is a result of reduced clearance (perhaps due to delay in development of glucuronosyltransferase and other enzyme systems involved in valproate elimination) as well as increased volume of distribution (in part due to decreased plasma protein binding). For example, in one study, the half-life in children under 10 days ranged from 10 to 67 hours compared to a range of 7 to 13 hours in children greater than 2 months.
- Children: Pediatric patients (i.e., between 3 months and 10 years) have 50% higher clearances expressed on weight (i.e., mL/min/kg) than do adults. Over the age of 10 years, children have pharmacokinetic parameters that approximate those of adults.
- Elderly: The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26). Intrinsic clearance is reduced by 39%; the free fraction is increased by 44%. Accordingly, the initial dosage should be reduced in the elderly.
- Effect of Sex: There are no differences in the body surface area adjusted unbound clearance between males and females (4.8 ±0.17 and 4.7±0.07 L/hr per 1.73 m2, respectively).
- Effect of Race: The effects of race on the kinetics of valproate have not been studied.
- Liver Disease: Liver disease impairs the capacity to eliminate valproate. In one study, the clearance of free valproate was decreased by 50% in 7 patients with cirrhosis and by 16% in 4 patients with acute hepatitis, compared with 6 healthy subjects. In that study, the half-life of valproate was increased from 12 to 18 hours. Liver disease is also associated with decreased albumin concentrations and larger unbound fractions (2 to 2.6 fold increase) of valproate. Accordingly, monitoring of total concentrations may be misleading since free concentrations may be substantially elevated in patients with hepatic disease whereas total concentrations may appear to be normal.
- Renal Disease: A slight reduction (27%) in the unbound clearance of valproate has been reported in patients with renal failure (creatinine clearance < 10 mL/minute); however, hemodialysis typically reduces valproate concentrations by about 20%. Therefore, no dosage adjustment appears to be necessary in patients with renal failure. Protein binding in these patients is substantially reduced; thus, monitoring total concentrations may be misleading.
- Tell patients not to stop taking valproic acid or change their dose without first talking to the health care provider and inform patients of the serious side effects.
- Serious liver damage that can cause death, especially in children younger than 2 years old can occur. The risk of getting this serious liver damage is more likely to happen within the first 6 months of treatment. Inform patients to call the health care provider right away if any of the following symptoms occur: nausea or vomiting that does not go away, loss of appetite, pain on the right side of the stomach, dark urine, swelling of the face, yellowing of the skin or the whites of the eyes.
- If taking valproic acid during pregnancy for any medical condition, the baby is at risk for serious birth defects and the child is at risk for having a lower IQ. All women of childbearing age should talk to the health care provider about using other possible treatments instead of valproic acid. If the decision is made to use valproic acid, effective birth control should be used. Advise patients to tell the health care provider right away if pregnancy occurs while taking valproic acid.
- Call the health care provider right away if any of the following symptoms occur: severe stomach pain that may be also felt in the back, nausea or vomiting that does not go away.
- Valproic acid may cause suicidal thoughts of actions in a very small number of people, about 1 in 500. Advise patients to call the health care provider right away if any of the following symptoms occur: thoughts about suicide or dying, attempts to commit suicide, new or worse depression, new or worse anxiety, felling agitated or restless; panic attacks, trouble sleeping, new or worse irritability, acting aggressive, being angry, or violent, acting on dangerous impulses, an extreme increase in activity and talking (mania), other unusual changes in behavior or mood.
- Inform patients not to take valproic acid if they have:
- Liver problems
- Have or think they have a genetic liver problem caused by a mitochondrial disorder
- Are allergic to divalproex sodium, valproic acid, sodium valproate, or any of the ingredients in Depakote or Depakene
- Have a genetic problem called urea cycle disorder
- Inform patients to tell the health care provider about all the medicines they take, including prescription and non-prescription medicines, vitamins, herbal supplements, and medicines that they take for a short period of time.
- Inform patients not to drink alcohol or take other medicines that make then sleepy or dizzy while taking valproic acid.
- Inform patients not to drive a car or operate dangerous machinery until they know how valproic acid will affect them.
- Inform patients that a fever associated with other organ system involvement (e.g., rash, lymphadenopathy) may be drug-related; instruct to report to physician.
Indications
Dosing (Adult)
Dosing (Pediatric)
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|