Trimethoprim Sulfamethoxazole (Bactrim, Septra): Drug Monograph
|
|---|
- Treatment of acute otitis media
- Treatment of acute exacerbations of chronic bronchitis (AECB)in adults
-
Treatment & prophylaxis of Pneumocystis jiroveci pneumonia(PJP)
-
Treatment of enteritis and urinary tract infections (UTIs)
-
Treatment of traveler's diarrhea (adults)
- Acute bacterial exacerbation of chronic bronchitis:
- One 800 mg/160 mg or two 400 mg/80 mg or 4 teaspoons of suspension every 12 hours for 14 days
- Pneumocystis jiroveci pneumonia (PJP):
- Treatment:
- Injection: 15-20 mg/kg/day (based on the trimethoprim component) given in 3 or 4 equally divided doses every 6-8 hours for up to 14 days
- Suspension/tablet: 75-100 mg/kg sulfamethoxazole and 15-20 mg/kg trimethoprim per 24 hours given in equally divided doses every 6 hours for 14-21 days
- Prophylaxis:
- Suspension/tablet: One 800 mg/160 mg tablet or 4 teaspoons of suspensiondaily
- Shigellosis:
- Injection: 8-10 mg/kg/day (based on the trimethoprim component) given in equally divided doses every 6, 8, or 12 hours for up to 5 days
- Suspension/tablet: One 800 mg/160 mg tablet or two 400 mg/80 mg tablets or 4 teaspoons (20 mL) of suspension every 12 hours for 5 days
- Traveler's diarrhea:
- Suspension/tablet: One 800 mg/160 mg tablet or two 400 mg/80 mg tablets or 4 teaspoons of suspension every 12 hours for 5 days
- Urinary tract infections:
- Injection (severe infections): 8-10 mg/kg/day (based on the trimethoprim component) given in equally divided doses every 6, 8 or 12 hours for up to 14 days
- Suspension/tablet: One 800 mg/160 mg tablet or two 400 mg/80 mg tablets or 4 teaspoons (20 mL) of suspension every 12 hours for 10-14 days
- Acute otitis media, ≥2 months of age:
- Use only when sulfamethoxazole/trimethoprim could offer some advantage over the use of a single antimicrobial agent
- Suspension/tablet: 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in 2 divided doses every 12 hours for 10 days
- PCP prophylaxis in immunosuppressed patients, ≥2 months of age:
- Suspension/tablet: 750 mg/m2/day sulfamethoxazole with 150 mg/m2/day trimethoprim given in equally divided doses twice a day, on 3 consecutive days/week.
- Not to exceed 1600 mg/day sulfamethoxazole and 320 mg/day trimethoprim
- PCP treatment, ≥2 months of age:
- Injection: 15-20 mg/kg/day (based on the trimethoprim component) given in 3 or 4 equally divided doses every 6-8 hours for up to 14 days
- Suspension/tablet: 75-100 mg/kg sulfamethoxazole and 15-20 mg/kg trimethoprim per 24 hours given in equally divided doses every 6 hours for 14-21 days
- Shigellosis, ≥2 months of age:
- Injection: 8-10 mg/kg/day (based on the trimethoprim component) given in equally divided doses every 6, 8, or 12 hours for up to 5 days
- Maximum: 60 mL/day
- Suspension/tablet: 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in 2 divided doses every 12 hours for 5 days
- Urinary tract infections, ≥2 months of age:
- Injection, severe infections: 8-10 mg/kg/day (based on the trimethoprim component) given in equally divided doses every 6, 8, or 12 hours for up to 14 days
- Maximum: 60 mL/day
- Suspension/tablet: 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in 2 divided doses every 12 hours for 10 days
- CrCl 15-30 mL/min:
- Injection/suspension/tablet: One-half the usual dosage
- CrCl <15 mL/min:
- Use not recommended
- Sulfamethoxazole-/Trimethoprim (SMX/TMP) Injection: 80 mg/16 mg/mL [5 mL, 10 mL]
- Suspension: sulfamethoxazole 200 mg/trimethoprim 40 mg in each 5 mL
- Tablets: 400 mg/80 mg (Bactrim)
- Double strength tablets: 800 mg/160 mg (Bactrim DS
- Patients showing marked liver parenchymal damage, blood dyscrasias, megaloblastic bone marrow or severe renal insufficiency, where repeated measurements of the plasma concentration cannot be performed
- Known hypersensitivity to the active ingredients or the excipients, or other sulfonamides
- Premature babies; not to be given during the first six weeks of life because of the risk of producing kernicterus. It should probably not be given to children under 3 months of age.
- Not for treatment of streptococcal pharyngitis. Patients with Group A b-hemolytic (Sp) streptococcal tonsillopharyngitis have a greater incidence of bacteriologic failure when treated with the drug than do those patients treated with penicillin as evidenced by failure to eradicate this organism from the tonsillopharyngeal area.
- Must not be given in combination with dofetilide
- Elderly - carries an increased risk of severe adverse reactions. The risk is particularly greater when complicating conditions exist (e.g., impaired kidney and/or liver function, or concomitant use of other drugs). Severe skin reactions, or generalized bone marrow suppression or a specific decrease in platelets (with or without purpura) are most frequently reported severe adverse reactions.
- Treatment of pneumocystis carinii pneumonitis in patients with AIDS - patients may not tolerate or respond to trimethoprim sulfamethoxazole in the same manner as non-AIDS patients. The incidence of side effects, particularly rash, fever, and leucopenia may be greatly increased.
- Glucose-6-phosphate dehydrogenase deficiency - hemolysis may occur and may be dose related. Do not give unless absolutely essential, and then only in minimal doses.
- Pseudomembranous colitis - may lead in very rare instances to the development of severe colitis as a result of colonization with C. difficile, a toxin-producing organism. If significant diarrhea occurs, discontinue treatment.
- Severe reactions - including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms (DRESS), fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Discontinue is a skin rash appears. Clinical signs such as rash, sore throat, fever, arthralgia, cough, shortness of breath, pallor, purpura or jaundice may be early indications of serious reactions.
- Patients with liver damage, renal damage, urinary obstruction, blood dyscrasias, allergies or bronchial asthma - make critical appraisal of benefit versus risk
- Patients with serious hematological disorders - do not give except under careful supervision
- Folate metabolism - with patients on long-term therapy who are predisposed to folate deficiency (i.e., the elderly, chronic alcoholics and rheumatoid arthritics), in malabsorption syndromes, malnutrition states, or during the treatment of epilepsy with anticonvulsant drugs such as phenytoin, primidone or barbiturates, regular blood counts are advisable.
- Superinfection
- Patients with porphyria or thyroid dysfunction - use caution
- Gastrointestinal disturbances - nausea, vomiting, anorexia
- Allergic skin reactions - rash, pruritus and urticaria
- Hematologic changes - particularly in the elderly
- Genitourinary - renal failure interstitial nephritis, BUN and serum creatinine elevation, toxic nephrosis with oliguria and anuria, and crystalluria
- Neurologic - aseptic meningitis convulsions, neuropathy, ataxia, vertigo, tinnitus, headache and uveitis
- Psychiatric - hallucinations, depression, apathy, nervousness
- Musculoskeletal - arthralgia, myalgia, and isolated cases of rhabdomyolysis
- Respiratory - pulmonary infiltrates
- Weakness, fatigue, insomnia
- Fungal infections - candidiasis
- Symptoms of sulfamethoxazole overdosage may include anorexia, colic, nausea, vomiting, dizziness, headache, drowsiness and unconsciousness. Pyrexia, hematuria and crystalluria may be noted. Blood dyscrasias and jaundice are potential late manifestations.
- Symptoms of acute overdosage with trimethoprim include nausea, vomiting, dizziness, headache, mental depression, confusion, and bone marrow depression.
- General supportive measures necessary. General principles of treatment include the prevention of further absorption, forcing of oral fluids, and the administration of IV fluids if urine output is low and renal function is normal.
- Monitor with blood counts and appropriate blood chemistries, including electrolytes.
- On cessation of therapy calcium folinate, 3 mg to 6 mg intramuscularly for five to seven days may be given to counteract the effects of trimethoprim on hematopoiesis.
- Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating the drug.
- Drugs transported by Organic Cation Transporter 2 (OCT2) (i.e., dofetilide, amantadine and memantine) - systemic exposure to these drugs may increase
- Dofetilide - causes elevated plasma levels of dofetilide and may cause serious ventricular arrhythmias associated with QT interval prolongation, including torsades de pointes. Do not co-administer
- Amiodarone - systemic exposure may increase
- Antivirals (amantadine/memantine) - may have increased risk of neurological adverse events such as delirium and myoclonus
- Paclitaxel - systemic exposure may increase
- Dapsone - systemic exposure may increase; potential for both pharmacokinetic and pharmacodynamic interactions
- Rifampicin - shortening of the plasma half-life of trimethoprim after a period of about one week
- Oral hypoglycemic agents (repaglinide rosiglitazone or pioglitazone) and sulfonylurea derivatives (glibenclamide, gliclazide, glipizide, chlorpropamide, and tolbutamide) - trimethoprim sulfamethoxazole potentiates the effect of these drugs; systemic exposure may increase. Monitor patients regularly for hypoglycemia.
- Anticoagulants (warfarin, aceocoumarol, phenprocoumon) - system exposure of these drugs may increase. Coagulation should be monitored.
- Antiepileptics (phenytoin) - systemic exposure of these drugs may increase. Be alert for excessive phenytoin effect.
- Cardiovascular agents (digoxin) - increased digoxin blood levels can occur, especially in elderly patients. Serum digoxin levels should be monitored.
- PABA - antagonize sulfamethoxazole. Increased sulfamethoxazole blood levels may occur in patients who are also receiving urinary acidifiers, oral anticoagulants, phenylbutazone, oxyphenbutazone, indomethacin, sulfinpyrazone, or salicylates.
- Clozapine - co-administration should be avoided
- Diuretics, primarily thiazides - an increased incidence of thrombocytopenia possible in elderly patients. Platelets should be monitored regularly.
- Antimalarials (pyrimethamine) - patients may develop megaloblastic anemia
- Antimetabolies (methotrexate) - cases of pancytopenia have been reported. The toxicity of methotrexate may increase, especially in the presence of risk factors such as old age, hypoalbuminemia, impaired renal function and decreased bone marrow reserve, and in patients receiving high doses of methotrexate.
- Antibacterials (sulfonamides) - can compete with protein binding and also with the renal transport of methotrexate, thus increasing the free methotrexate fraction and the systemic exposure to methotrexate, or increasing the antibacterial activity of sulfamethoxazole.
- Antithyroid agents, diuretics, oral hypoglycemic drugs - cross sensitivities may exist
- Antidepressants (tricyclic antidepressants) - the efficacy of these drugs can decrease
- Antivirals (zidovudine) - known to induce hematological abnormalities. Potential for an additive pharmacodynamic effect. Monitor patients for hematological toxicity. Dosage adjustment may be needed.
- Immunosuppressants (azathioprine mercaptopurine) - may increase the risk of hematological adverse events, particularly in patients who receive trimethoprim sulfamethoxazole for an extended period, or who are at an increased risk of folic acid deficiency.
- Cyclosporine - a reversible deterioration of renal function may occur
- Angiotensin converting-enzyme inhibitors and angiotensin receptor blockers - caution when co-administered due to the potassium-sparing effects. Frequent monitoring of serum potassium is recommended, particularly in patients with underlying potassium disorders, renal insufficiency, or patients receiving a high dose of trimethoprim sulfamethoxazole.
- Pregnancy: Injection/suspension: Pregnancy Category C. Tablet: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: Both trimethoprim and sulfamethoxazole are excreted in breast milk at concentrations comparable or somewhat lower than that in the blood. It is recommended that the possible risks should be balanced against the expected therapeutic effect. Consideration should be made of the infant's age. A folate supplement may be considered with prolonged high dose of the drug.
- Renal Impairment: CrCl >25mL/min: same dosage as for adults with normal kidney functions. CrCl 15-25 mL/min: One half the usual dosage. CrCl <15 mL/min: use not recommended
- Hepatic Impairment: None
- Pediatric Patients: Contraindicated for infants younger than 2 months of age.
- Geriatric Patients: There may be an increased risk of severe adverse reactions in elderly patients, particularly when complicating conditions exist, e.g., impaired kidney and/or liver function, possible folate deficiency, or concomitant use of other drugs.
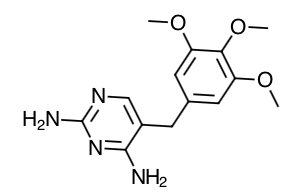
- Trimethoprim Structure
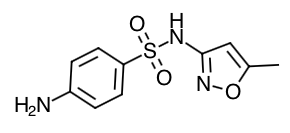
- Sulfamethoxaole Structure
- Scientific Name: Trimethoprim: 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine Sulfamethoxazole: 3-(4-aminobenzenesulfonamido)-5-methylisoxazole
- Empirical Formula: Trimethoprim: C14H18N4O3 Sulfamethoxazole: C10H11N3O3S
- Molecular Weight: Trimethoprim: 290.3 Sulfamethoxazole: 253.28
- Sulfamethoxazole inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid. Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus it blocks two consecutive steps in the biosynthesis of nucleic acids and proteins essential to bacteria.
- Trimethoprim sulfamethoxazole is effective against a wide range of Gram-negative and Gram-positive organisms; for example, E. coli, Neisseria, Salmonella, Klebsiella-Enterobacter, Shigella, Vibrio cholerae and Bordetella pertussis, Streptococcus, Staphylococcus, Pneumococcus. It is usually active against the problem organisms Haemophilus influenzae and Proteus.
- Trimethoprim sulfamethoxazole is also active against the protozoan Pneumocystis carinii (see special dosage instructions). It is not active against Mycobacterium tuberculosis and Treponema pallidum. Pseudomonas aeruginosa is frequently insensitive.
- Absorption: Trimethoprim sulfamethoxazole is rapidly absorbed on oral administration reaching peak blood levels after 1 to 4 hours, which correspond to those achieved when each component is given alone. The mean serum half-lives of trimethoprim and sulfamethoxazole are 10 hours and 8-10 hours, respectively.
- Distribution: The volume of distribution of trimethoprim is about 130 liters and that of sulfamethoxazole is about 20 liters. At the above concentrations, about 42 - 45% of trimethoprim and 66% of sulfamethoxazole is bound to plasma proteins. The free forms of trimethoprim and sulfamethoxazole are considered to be the therapeutically active forms. Studies in both animals and man have shown that diffusion of trimethoprim sulfamethoxazole into the tissue is good. Large amounts of trimethoprim and smaller amounts of sulfamethoxazole pass from the bloodstream into the interstitial fluid and other extravascular body fluids. In humans, trimethoprim and sulfamethoxazole were detected in the fetal placenta, umbilical cord blood, amniotic fluid and fetal tissues (liver, lung), indicating placental transfer of both drugs.
- Metabolism: Approximately 50 - 70% of the trimethoprim dose and 10 - 30% are excreted unchanged. The principal trimethoprim metabolites are 1- and 3-oxides and the 3'- and 4'- hydroxy derivatives; some metabolites are active. Sulfamethoxazole is metabolized in the liver, predominantly by N4- acetylation and to a lesser extent by glucuronide conjugation; the metabolites are inactive.
- Elimination: The elimination half-lives of the two components are very similar (a mean of 10 hours for trimethoprim and 11 hours for sulfamethoxazole). Both substances, as well as their metabolites, are eliminated almost entirely by the kidneys through both glomerular filtration and tubular secretion, giving urine concentrations of both active substances considerably higher than the concentration in the blood. A small part of the substances is eliminated via the feces.
-
Patients should be counseled that antibacterial drugs, including trimethoprim sulfamethoxazole, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold).
-
Patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of the therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by the medication or other antibacterial drugs in the future.
-
Patients should be counseled to maintain an adequate fluid intake in order to prevent crystalluria and stone formation.
-
Advise patients that diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without c=stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
-
Patients should be informed that complete blood counts should be done frequently in patients receiving trimethoprim sulfamethoxazole. If a significant reduction in the count of any formed blood element is noted, the drug should be discontinued.
-
Urinalyses with careful microscopic examination and renal function tests should be performed during therapy, particularly for those patients with impaired renal function.
Indications
Dosing (Adult)
General Notes: Injection: Administer by IV infusion over 60-90 minutes; avoid rapid infusion or bolus injection. Do not mix with other drugs or solution. Suspension: Shake well before using. Refer to PI for specific weight-dose recommendations for suspension/tablet use in pediatrics. In acute infections, should be given for at least five days or until the patient has been symptom-free for two days.
Dosing (Pediatric)
Renal Dosing
Dosage Forms
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





