Tirofiban (Aggrastat): Drug Monograph
|
|---|
- To reduce the rate of thrombotic cardiovascular events (combined endpoint of death, myocardial infarction, or refractory ischemia/repeat cardiac procedure) in patients with non-ST elevation acute coronary syndrome (NSTE-ACS)
- 25 mcg/kg IV within 5 minutes, then 0.15 mcg/kg/min for up to 18 hours
- Refer to PI for dosing by weight and CrCl
- CrCl ≤60 mL/min:
- 25 mcg/kg IV within 5 minutes, then 0.075 mcg/kg/min for up to 18 hours
- Refer to PI for dosing by weight and CrCl
- Known hypersensitivity to any component of tirofiban
- History of thrombocytopenia with prior exposure to tirofiban
- Active internal bleeding, or history of bleeding diathesis, major surgical procedure or severe physical trauma within the previous month
- Serious bleeding - if bleeding cannot be controlled, discontinue treatment
- Thrombocytopenia - discontinue tirofiban and heparin
- Most frequently reported manifestation was bleeding, primarily minor mucocutaneous bleeding events and minor bleeding at the sites of cardiac catheterization.
- Should be treated by assessment of the patient's clinical condition and cessation or adjustment of the drug infusion as appropriate.
- Can be removed by hemodialysis.
- Antiplatelet agents, thrombolytics, heparin, or aspirin - increases the risk of bleeding
- Pregnancy: Pregnancy Category B
- Labor and Delivery: None
- Nursing Mothers: It is not known whether tirofiban is excreted in human milk. Discontinue nursing or discontinue tirofiban.
- Renal Impairment: Reduce the dose in patients with severe renal insufficiency. Safety and efficacy have not been established in patients on hemodialysis.
- Hepatic Impairment: None
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: No dose adjustment is recommended.
- It is not known whether tirofiban is excreted in human milk. Discontinue nursing or discontinue tirofiban.
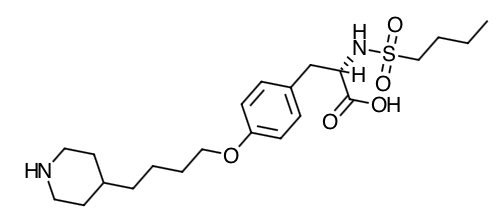
- Scientific Name: N(butylsulfonyl)-O-[4-(4-piperidinyl)butyl]-L-tyrosine monohydrochloride monohydrate.
- Empirical Formula: C22H36N2O5S.HCl.H2O
- Molecular Weight: 495.08
- Tirofiban is a reversible antagonist of fibrinogen binding to the GP IIb/IIla receptor, the major platelet surface receptor involved in platelet aggregation. When administered intravenously, tirofiban inhibits ex vivo platelet aggregation in a dose- and concentration-dependent manner.
- When given according to the PRISM-PLUS regimen of 0.4 mcg/kg/min over 30 minutes followed by a 0.1 mcg/kg/min maintenance infusion, >90% inhibition of platelet aggregation is attained by the end of the 30-minute infusion. When given according to the recommended regimen of 25 mcg/ kg over 3 min followed by a 0.15 mcg/kg/min maintenance infusion, >90% inhibition of platelet aggregation is attained within 10 minutes. Platelet aggregation inhibition is reversible following cessation of the infusion of tirofiban.
- Tirofiban inhibits platelet function, as demonstrated by its ability to inhibit ex vivo adenosine phosphate (ADP)-induced platelet aggregation and prolong bleeding time in healthy subjects and patients with coronary artery disease. The time course of inhibition parallels the plasma concentration profile of the drug.
- Following discontinuation of an infusion of tirofiban 0.10 mcg/kg/ min, ex vivo platelet aggregation returns to near baseline in 4 to 8 hours in approximately 90% of patients with coronary artery disease. The addition of heparin to this regimen does not significantly alter the percentage of subjects with >70% inhibition of platelet aggregation (IPA), but does increase the average bleeding time, as well as the number of patients with bleeding times prolonged to >30 minutes. Similar platelet aggregation recovery rates are observed following discontinuation of a 0.15 mcg/kg/ min infusion.
- Absorption: Tirofiban has a half-life of approximately 2 hours.
- Distribution: Tirofiban is not highly bound to plasma proteins and protein binding is concentration independent over the range of 0.01 to 25 mcg/mL. The unbound fraction in human plasma is 35%. The steady state volume of distribution of tirofiban ranges from 22 to 42 liters. In healthy subjects, the plasma clearance of tirofiban ranges from 213 to 314 mL/min. Renal clearance accounts for 39 to 69% of plasma clearance.
- Metabolism: Metabolism appears to be limited.
- Elimination: Tirofiban is cleared from the plasma largely by renal excretion, with about 65% of an administered dose appearing in urine and about 25% in feces, both largely as unchanged tirofiban.
- Special Populations:
- There is no effect on clearance of tirofiban by sex, race, age, or hepatic impairment.
- Renal Insufficiency: Plasma clearance of tirofiban is decreased about 40% in subjects with creatinine clearance <60 mL/min and >50% in patients with creatinine clearance <30 mL/min, including patients requiring hemodialysis. Tirofiban is removed by hemodialysis.
- Advise patients to watch closely for any signs of bleeding or bruising and to report these to their health care provider when they occur.
- Advise patients to discuss with their health care provider their use of any other medications, including over-the-counter or herbal products prior to tirofiban use.
Indications
Dosing (Adult)
General Notes: Do not administer through the same IV line as diazepam. May administer in the same IV line as atropine sulfate, dobutamine, dopamine, epinephrine hydrochloride, famotidine injection, furosemide, lidocaine, midazolam HCL, morphine sulfate, nitroglycerin, potassium chloride, and propranolol HCL. Dl not administer through the same IV line as diazepam. Do not add other drugs or remove solution directly from the bag with a syringe; do not use plastic containers in series connections
Renal Dosing
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

