Ticagrelor (Brilinta): Drug Monograph
|
|---|
- Reduce the rate of thromobotic cardiovascular events in patients with acute coronary syndrome (ACS) (unstable angina, non-ST elevation MI, or ST elevation MI)
- General Notes:
- Take with or without food.
- If unable to swallow tablet whole, tablet can be crushed, mixed with water, and drunk immediately; the glass should be refilled with water, stirred, and the contents drunk. The mixture can be also administered via a NG tube (≥CH8); it is important to flush the NG tube through with water after administration of the mixture
- Acute Coronary Syndrome:
- Initiate treatment with 180 mg (two 90 mg tablets) with aspirin (ASA) (usually 325 mg) by mouth or NG tube
- Maintenance: 90 mg by mouth twice a day + ASA (75-100 mg/day) x 12 months; after 12 months reduce ticagrelor to 60 mg by mouth twice a day.
- Notes:
- Patients who have received a loading dose of clopidogrel, then start ticagrelor beginning 24 hours after the last dose of clopidogrel and use a dose of 90 mg by mouth twice a day.
- If a dose is missed, take one 90 mg tablet (next dose) at its scheduled time
- None
- Can cause significant, sometimes fatal bleeding.
- Do not use in patients with active pathological bleeding or a history of intracranial hemorrhage.
- Do not start ticagrelor in patients planned to undergo urgent coronary artery bypass graft surgery. Discontinue at least 5 days prior to any surgery, when possible.
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention, CABG, or other surgical procedures in the settling of ticagrelor.
- If possible, manage bleeding without discontinuing ticagrelor. Stopping increases the risk of subsequent cardiovascular events.
- Maintenance doses of aspirin above 100 mg reduce the effectiveness of ticagrelor and should be avoided.
- History of intracranial hemorrhage
- Active pathological bleeding
- Severe hepatic impairment
- Hypersensitivity to ticagrelor or any component of the product
- Risk of bleeding
- Decreased effectiveness of ticagrelor when used with maintenance doses of aspirin above
- 100 mg
- Hepatic impairment
- Dyspnea reported more frequently with ticagrelor
- Premature discontinuation of ticagrelor increases the risk of myocardial infarction, stent thrombosis, and death.
- Treatment of overdose should follow local standard medical practice.
- Bleeding is the expected pharmacologic effect of overdosing.
- Other effects may include gastrointestinal effects or ventricular pauses.
- Monitor the ECG.
- CYP450 Enzymes:
- Major CYP3A Substrate
- Note:
- Does not inhibit or induce any of the CYP450 enzymes
- Simvastatin or lovastatin - more than 40 mg per day may increase risk of statin-related adverse effects.
- Monitor digoxin levels with initiation or any change in ticagrelor
- Transporters:
- Weak inhibitor of P-gp
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is unknown if ticagrelor is excreted in human milk. Discontinue nursing or discontinue drug.
- Renal Impairment: No dosage adjustment is needed.
- Hepatic Impairment: Contraindicated for use in patients with severe hepatic impairment. Its use should be considered carefully in patients with moderate hepatic impairment.
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: No overall differences in safety or effectiveness between older and younger patients.
- It is unknown if ticagrelor is excreted in human milk. Discontinue nursing or discontinue drug.
-
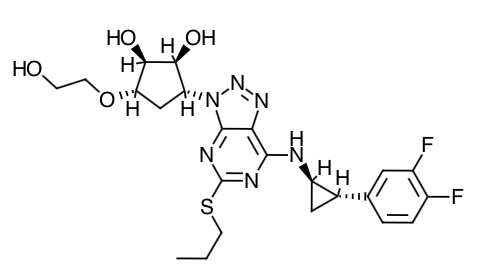
Scientific Name: 1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-3H-[1,2,3]-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol.
-
Empirical Formula: C23H28F2N6O4S
-
Molecular Weight: 522.57
- Ticagrelor and its active metabolite both reversibly inhibit the platelet P2Y12ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.
- The inhibition of platelet aggregation (PA) by ticagrelor and clopidogrel was compared in a 6 week study examining both acute and chronic platelet inhibition effects in response to 20 µM ADP as the platelet aggregation agonist.
- The onset of IPA was evaluated on Day 1 of the study following loading doses of 180 mg ticagrelor or 600 mg clopidogrel. IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours.
- The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 µM ADP.
- Mean maximum IPA following the last dose of ticagrelor was
88% and 62% for clopidogrel. After 24
hours, IPA in the ticagrelor group (58%) was similar to IPA in clopidogrel
group (52%), indicating that patients who miss a dose of ticagrelor would still
maintain IPA similar to the trough IPA of patients treated with
clopidogrel. After 5 days, IPA in the
ticagrelor group was similar to IPA in the placebo group. It is not know how either bleeding risk or
thrombotic risk track with IPA, for either ticagrelor or clopidogrel.
- Transitioning from clopidogrel to ticagrelor resulted in an absolute IPA increase of 26.4% and from ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to ticagrelor without interruption of antiplatelet effect.
- Absorption: The mean absolute bioavailability of ticagrelor is about 36%, (range 30%-42%).
- Ingestion of a high-fat meal had no effect on ticagrelor Cmax, but resulted in a 21% increase in AUC.
- Absorption of ticagrelor occurs with a median Tmax of 1.5 h (range 1.0-4.0).
- Ticagrelor can be taken with or without food.
- Distribution:
- The steady state volume of distribution of ticagrelor is 88 L.
- Extensively bound to human plasma proteins (>99%).
- Metabolism:
- CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite.
- Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors.
- Elimination:
- The primary route of ticagrelor elimination is hepatic metabolism.
- The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion.
- The mean t1/2 is approximately 7 hours for ticagrelor and 9 hours for the active metabolite.
- Special Population: The effects of age, gender, ethnicity, renal impairment and mild hepatic impairment on the pharmacokinetics of ticagrelor are modest and do not require dose adjustment.
- Tell patients to take ticagrelor exactly as prescribed and not to discontinue ticagrelor without discussing it with the prescribing physician.
- Tell patients daily doses of aspirin should not exceed 100 mg and to avoid taking any other medications that contain aspirin.
- Inform patients that they will bleed and bruise more easily, will take longer than usual to stop bleeding, and should report any unanticipated, prolonged or excessive bleeding, or blood in their stool or urine.
- Instruct patients to contact their doctor if they experience unexpected shortness of breath, especially if severe.
- Instruct patients to inform physicians and dentists that they are taking ticagrelor before any surgery or dental procedure and to talk to the prescribing physician before stopping therapy.
- Advise patients to list all prescription medications, over-the-counter medications or dietary supplements they are taking or plan to take so the physician knows about other treatments that may affect bleeding risk (e.g. warfarin, heparin).
Indications
Dosing
(Adult)
(Pediatrics)
Black Box Warnings
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|