Telmisartan (Micardis): Drug Monograph
|
|---|
- Treatment of hypertension alone or in combination with other antihypertensive agents
- Reduction of risk of MI, stroke, or death from cardiovascular (CV) causes in patients ≥55 years of age at high risk of developing major CV events who are unable to take ACE inhibitors
- Hypertension:
- 40 mg by mouth with or without food once daily
- Range: 40-80 mg/day
- Note: May add diuretic if blood pressure not controlled with 80 mg
- Cardiovascular Risk Reduction (Myocardial Infarction, Stroke, Cardiovascular Death):
- 80 mg by mouth with or without food once daily
- Impairment, including biliary obstructive disorders:
- Start at low doses and titrate slowly
- When pregnancy is detected, discontinue telmisartan as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
- Known hypersensitivity (e.g., anaphylaxis or angioedema) to telmisartan or any other component of this product
- Do not co-administer aliskiren with telmisartan in patients with diabetes
- Avoid fetal or neonatal exposure
- Hypotension: correct any volume or salt depletion before initiating therapy. Observe for signs and symptoms of hypotension
- Monitor carefully in patients with impaired hepatic or renal function
- Avoid concomitant use of an ACE inhibitor and angiotensin receptor blocker
- Hypertension:
- Back pain
- Sinusitis
- Diarrhea
- CV risk reduction:
- Intermittent claudication
- Skin ulcer
- Limited data are available with regard to overdosage in humans.
- Most likely manifestation would be hypotension, dizziness and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation.
- If symptomatic hypotension should occur, supportive treatment should be instituted.
- Telmisartan is not removed by hemodialysis.
- NSAIDs - increased risk of renal impairment and loss of antihypertensive effect
- Aliskiren - do not co-administer in patients with diabetes
- Digoxin - monitor digoxin levels when initiating adjusting, and discontinuing telmisartan for the purpose of keeping the digoxin level within the therapeutic range
- Lithium - monitor serum lithium levels during concomitant use
- Pregnancy: Pregnancy Category D
- Labor and Delivery: None
- Nursing Mothers: Choose to discontinue nursing or drug
- Renal Impairment: None
- Hepatic Impairment: Monitor carefully and uptitrate slowly in patients with biliary obstructive disorders or hepatic insufficiency.
- Pediatric Patients:
- Safety and effectiveness have not been established.
- Neonates with a history of in utero exposure to telmisartan:
- If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
- Geriatric Patients: No overall difference in efficacy or safety vs younger patients, but greater sensitivity of some older individuals cannot be ruled out.
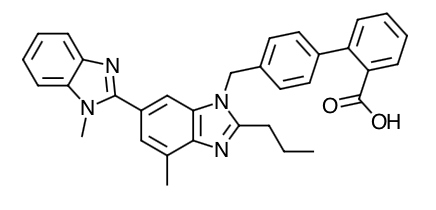
- Scientific Name: 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid
- Empirical Formula: C33H30N4O2
- Molecular Weight: 514.63
- Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Telmisartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
- There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Telmisartan has much greater affinity (>3,000 fold) for the AT1 receptor than for the AT2 receptor.
- Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because telmisartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Telmisartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
- Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of telmisartan on blood pressure.
- In normal volunteers, a dose of telmisartan 80 mg inhibited the pressor response to an intravenous infusion of angiotensin II by about 90% at peak plasma concentrations with approximately 40% inhibition persisting for 24 hours.
- Plasma concentration of angiotensin II and plasma renin activity (PRA) increased in a dose-dependent manner after single administration of telmisartan to healthy subjects and repeated administration to hypertensive patients. The once-daily administration of up to 80 mg telmisartan to healthy subjects did not influence plasma aldosterone concentrations. In multiple dose studies with hypertensive patients, there were no clinically significant changes in electrolytes (serum potassium or sodium), or in metabolic function (including serum levels of cholesterol, triglycerides, HDL, LDL, glucose, or uric acid).
- In 30 hypertensive patients with normal renal function treated for 8 weeks with telmisartan 80 mg or telmisartan 80 mg in combination with hydrochlorothiazide 12.5 mg, there were no clinically significant changes from baseline in renal blood flow, glomerular filtration rate, filtration fraction, renovascular resistance, or creatinine clearance.
-
Absorption: Following oral administration, peak concentrations (Cmax) of telmisartan are reached in 0.5 to 1 hour after dosing. Food slightly reduces the bioavailability of telmisartan, with a reduction in the area under the plasma concentration-time curve (AUC) of about 6% with the 40 mg tablet and about 20% after a 160 mg dose. The absolute bioavailability of telmisartan is dose dependent. At 40 and 160 mg the bioavailability was 42% and 58%, respectively. The pharmacokinetics of orally administered telmisartan are nonlinear over the dose range 20 to 160 mg, with greater than proportional increases of plasma concentrations (Cmax and AUC) with increasing doses. Telmisartan shows bi-exponential decay kinetics with a terminal elimination half-life of approximately 24 hours. Trough plasma concentrations of telmisartan with once daily dosing are about 10% to 25% of peak plasma concentrations. Telmisartan has an accumulation index in plasma of 1.5 to 2.0 upon repeated once daily dosing.
-
Distribution: Telmisartan is highly bound to plasma proteins (>99.5%), mainly albumin and α1-acid glycoprotein. Plasma protein binding is constant over the concentration range achieved with recommended doses. The volume of distribution for telmisartan is approximately 500 liters indicating additional tissue binding.
-
Metabolism: Telmisartan is metabolized by conjugation to form a pharmacologically inactive acyl glucuronide; the glucuronide of the parent compound is the only metabolite that has been identified in human plasma and urine. After a single dose, the glucuronide represents approximately 11% of the measured radioactivity in plasma. The cytochrome P450 isoenzymes are not involved in the metabolism of telmisartan. Total plasma clearance of telmisartan is >800 mL/min. Terminal half-life and total clearance appear to be independent of dose.
-
Elimination: Following either intravenous or oral administration of 14C-labeled telmisartan most of the administered dose (>97%) was eliminated unchanged in feces via biliary excretion; only minute amounts were found in the urine (0.91% and 0.49% of total radioactivity, respectively).
-
Special Populations
-
Renal Insufficiency: No dosage adjustment is necessary in patients with decreased renal function. Telmisartan is not removed from blood by hemofiltration.
-
Hepatic Insufficiency: In patients with hepatic insufficiency, plasma concentrations of telmisartan are increased, and absolute bioavailability approaches 100%.
-
Gender: Plasma concentrations of telmisartan are generally 2 to 3 times higher in females than in males. In clinical trials, however, no significant increases in blood pressure response or in the incidence of orthostatic hypotension were found in women. No dosage adjustment is necessary.
-
Geriatric Patients: The pharmacokinetics of telmisartan do not differ between the elderly and those younger than 65 years.
-
Pediatric Patients: Telmisartan pharmacokinetics have not been investigated in patients <18 years of age.
Indications
Dosing (Adult)
General Dosing & Administration Notes: May be administered with or without food. When used for CV risk reduction, monitoring of blood pressure is recommended, and if appropriate, adjustment of medications that lower blood pressure may be necessary.
Hepatic Dosing
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
MESH Terms & Keywords
|
|---|
|





