Tedizolid (Sivextro): Drug Monograph
|
|---|
- Treatment of acute bacterial skin and skin structure infections (ABSSSIs) caused by susceptible isolates of gram-positive microorganisms
- Administer with or without food.
- No dose adjustment is necessary when changing from IV to oral route.
- If patients miss a dose, they should take it as soon as possible anytime up to 8 hours prior to their next scheduled dose; if <8 hours remain before the next dose, wait until their next scheduled dose.
- Preparation of the IV solution: Reconstitute the vial with 4 mL of SWFl. Gently swirl the contents and let the vial stand until the cake has completely dissolved and any foam disperses; if necessary, invert the vial to dissolve any remaining powder and swirl gently to prevent foaming. Tilt the upright vial and insert a syringe with appropriately sized needle into the bottom corner of the vial and remove 4 mL of the reconstituted solution; do not invert the vial during extraction. The reconstituted solution must b e further diluted in 250 mL of 0.9% NaCl injection. Slowly inject the 4 mL of reconstituted solution into a 250 mL bag of the NaCl injection ad invert the bag gently to mix; do not shake the bag.
- Administer as IV infusion only. Incompatibles: Any solution containing divalent cations (e.g., Ca2+, Mg2+), including lactated Ringer's Injection and Hartmann's solution.
- Do not add other IV substances, additives, or other medications to the single-use vials or infuse simultaneously; if the same IV line is used for sequential infusion of several different drugs, the line should be flushed before and aft4er infusion of tedizolid phosphate with 0.9% NaCl injection
- Skin and skin structure infections:
- Parenteral: 200 mg IV over 1 hour infusion once daily x 6 days
- Oral: 200 mg by mouth once daily x 6 days
- Patients with neutropenia - the safety and efficacy have not been adequately evaluated. The antibacterial activity of tedizolid may be reduced in the absence of granulocytes; consider alternative therapies in neutropenic patients
- Clostridium difficile-associated diarrhea - evaluate if diarrhea occurs
- Development of drug-resistant bacteria
- Discontinue drug and give general supportive treatment
- Hemodialysis does not result in meaningful removal of the drug from systemic circulation
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether tedizolid is excreted in human milk; caution should be exercised when administered to a nursing woman
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Safety and effectiveness have not been established
- Geriatric Patients: No overall differences in pharmacokinetics observed between elderly and younger subjects
- It is not known whether tedizolid is excreted in human milk; caution should be exercised when administered to a nursing woman
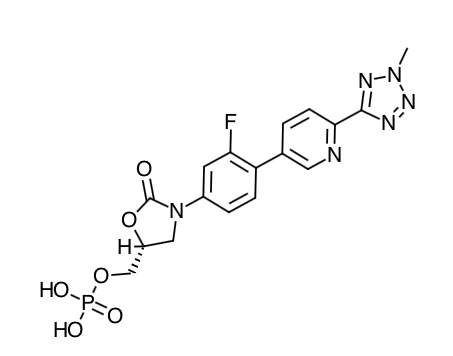
- Scientific Name: [(5R)-(3-{3-Fluoro-4-[6-(2-methyl-2H-tetrazol- 5-yl) pyridin-3-yl]phenyl}-2- oxooxazolidin- 5-yl]methyl hydrogen phosphate
- Empirical Formula: C17H16FN6O6P
- Molecular Weight: 450.32
- Tedizolid phosphate is the prodrug of tedizolid, an antibacterial agent. Inhibits bacterial protein synthesis by binding to the 50@ subunit of the bacterial ribosome
- The AUC/minimum inhibitory concentration (MIC) was shown to best correlate with tedizolid activity in animal infection models.
- In the mouse thigh infection model of S. aureus, antistaphylococcal killing activity was impacted by the presence of granulocytes. In granulocytopenic mice (neutrophil count <100 cells/mL), bacterial stasis was achieved at a human-equivalent dose of approximately 2000 mg/day; whereas, in non-granulocytopenic animals, stasis was achieved at a human-equivalent dose of approximately 100 mg/day. The safety and efficacy of tedizolid for the treatment of neutropenic patients (neutrophil counts <1000 cells/mm) have not been evaluated.
- Cardiac Electrophysiology: In a randomized, positive- and placebo-controlled crossover thorough QTc study, 48 enrolled subjects were administered a single oral dose of tedizolid at a therapeutic dose of 200 mg, tedizolid at a supratherapeutic dose of 1200 mg, placebo, and a positive control; no significant effects of tedizolid on heart rate, electrocardiogram morphology, PR, QRS, or QT interval were detected. Therefore, it does not affect cardiac repolarization.
- Absorption: Peak plasma tedizolid concentrations are achieved within approximately 3 hours following oral administration under fasting conditions or at the end of the 1-hour intravenous infusion of tedizolid phosphate. The absolute bioavailability is approximately 91% and no dosage adjustment is necessary between intravenous and oral administration. Tedizolid phosphate (oral) may be administered with or without food as total systemic exposure (AUC0-∞) is unchanged between fasted and fed (high-fat, high-calorie) conditions.
- Distribution: Protein binding of tedizolid to human plasma proteins is approximately 70 to 90%. The mean steady state volume of distribution of tedizolid in healthy adults following a single intravenous dose of tedizolid phosphate 200 mg ranged from 67 to 80 L (approximately twice total body water). Tedizolid penetrates into the interstitial space fluid of adipose and skeletal muscle tissue with exposure similar to free drug exposure in plasma
- Metabolism: Other than tedizolid, which accounts for approximately 95% of the total radiocarbon AUC in plasma, there are no other significant circulating metabolites in humans. There was no degradation of tedizolid in human liver microsomes indicating tedizolid is unlikely to be a substrate for hepatic CYP450 enzymes.
- Elimination: Following single oral administration of 14C-labeled tedizolid phosphate under fasted conditions, the majority of elimination occurred via the liver, with 82% of the radioactive dose recovered in feces and 18% in urine, primarily as a non-circulating and microbiologically inactive sulfate conjugate. Most of the elimination of tedizolid (>85%) occurs within 96 hours. Less than 3% of the tedizolid phosphate-administered dose is excreted in feces and urine as unchanged tedizolid.
- Specific Populations:
- Based on the population pharmacokinetic analysis, there are no clinically relevant demographic or clinical patient factors (including age, gender, race, ethnicity, weight, body mass index, and measures of renal or liver function) that impact the pharmacokinetics of tedizolid.
- Hepatic Impairment: Following administration of a single 200 mg oral dose, no clinically meaningful changes in mean tedizolid Cmax and AUC0-∞) were observed in patients with moderate (n=8) or severe (n=8) hepatic impairment (Child-Pugh Class B and C) compared to 8 matched healthy control subjects. No dose adjustment is necessary for patients with hepatic impairment.
- Renal Impairment: Following administration of a single 200 mg intravenous dose to 8 subjects with severe renal impairment defined as eGFR <30 mL/min/1.73 m2, the Cmax was essentially unchanged and AUC0-∞ was decreased by less than 10% compared to 8 matched healthy control subjects. Hemodialysis does not result in meaningful removal of tedizolid from systemic circulation, as assessed in subjects with end-stage renal disease (eGFR <15 mL/min/1.73 m2). No dosage adjustment is necessary in patients with renal impairment or patients on hemodialysis.
- Geriatric Patients: The pharmacokinetics of tedizolid were evaluated in a Phase 1 study conducted in elderly healthy volunteers (age 65 years and older, with at least 5 subjects at least 75 years old; n=14) compared to younger control subjects (25 to 45 years old; n=14) following administration of a single oral dose of 200 mg. There were no clinically meaningful differences in tedizolid Cmax and AUC0-∞ between elderly subjects and younger control subjects. No dosage adjustment is necessary in elderly patients.
- Gender: The impact of gender on the pharmacokinetics of tedizolid was evaluated in clinical trials of healthy males and females and in a population pharmacokinetics analysis. The pharmacokinetics of tedizolid were similar in males and females. No dosage adjustment is necessary based on gender.
- Drug Interaction Studies:
- Drug Metabolizing Enzymes: Transformation via Phase 1 hepatic oxidative metabolism is not a significant pathway for elimination of tedizolid. Neither Sivextro nor tedizolid detectably inhibited or induced the metabolism of selected CYP enzyme substrates. No potential drug interactions with tedizolid were identified in in vitro CYP inhibition or induction studies. These results suggest that drug-drug interactions based on oxidative metabolism are unlikely.
- Membrane Transporters: The potential for tedizolid or tedizolid phosphate to inhibit transport of probe substrates of important drug uptake (OAT1, OAT3, OATP1B1, OATP1B3, OCT1, and OCT2) and efflux transporters (P-gp and ABCG2 [also known as BCRP]) was tested in vitro. No clinically significant inhibition of any transporter was observed at tedizolid circulating plasma concentrations up to the Cmax.
- Monoamine Oxidase Inhibition: Tedizolid is a reversible inhibitor of monoamine oxidase (MAO) in vitro. The interaction with MAO inhibitors could not be evaluated in Phase 2 and 3 trials, as subjects taking such medications were excluded from the trials.
- Adrenergic Agents: Two placebo-controlled crossover studies were conducted to assess the potential of 200 mg oral tedizolid at steady state to enhance pressor responses to pseudoephedrine and tyramine in healthy individuals. No meaningful changes in blood pressure or heart rate were seen with pseudoephedrine. The median tyramine dose required to cause an increase in systolic blood pressure of ≥30 mmHg from pre-dose baseline was 325 mg with tedizolid compared to 425 mg with placebo. Palpitations were reported in 21/29 (72.4%) subjects exposed to tedizolid compared to 13/28 (46.4%) exposed to placebo in the tyramine challenge study.
- Serotonergic Agents: Serotonergic effects at doses of tedizolid phosphate up to 30-fold above the human equivalent dose did not differ from vehicle control in a mouse model that predicts serotonergic activity. In Phase 3 trials, subjects taking serotonergic agents including antidepressants such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, and serotonin 5-hydroxytryptamine (5-HT1) receptor agonists (triptans), meperidine, or buspirone were excluded.
- Inform patients that the drug may be taken with or without food and without any dietary restrictions
- Patients should be informed that tedizolid should only be used to treat bacterial infections, not viral infections (e.g., the common cold). The medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by tedizolid or other antibacterial drugs in the future.
- Patients should be informed that if they miss a dose, they should take the dose as soon as possible anytime up to 8 hours prior to their next scheduled dose. If less than 8 hours remain before the next dose, then they should wait until their net schedule dose.
- Patients should be informed that diarrhea is a common problem caused by antibacterial drugs and usually resolves when the drug is discontinued. Patients may develop frequent watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic and may be a sign of a more serious intestinal infection. If this occurs, patients should contact their healthcare provider as soon as possible.
Indications
Dosing (Adult)
General Dosing & Administration Notes:
Warnings
Overdose
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

