Sirolimus (Rapamune): Drug Monograph
|
|---|
- Prophylaxis of organ rejection in patients ≥13 years of age receiving renal transplants
- Treatment of patients with lymphangioleiomyomatosis
- General Dosing & Administration Notes:
- Administer once daily; give consistently with or without food.
- Tablet: Do not crush, chew, or split.
- Solution: Once the bottle is opened use within 1 month. Contains polysorbate 80, which is known to increase the rate of di-(2-ethylhexyl) phthalate extraction from polyvinyl chloride.
- Instructions for dilution: Use amber dose syringe to withdraw the prescribed amount of solution from bottle. Empty the correct amount of sirolimus from syringe into only a glass or plastic container holding at least 2 oz (1.4 cup, 60 mL) of water or orange juice; no other liquids, including grapefruit juice, should be used for dilution. Stir vigorously and drink at once. Refill the container with an additional volume (minimum of 4 oz [1/2 cup, 120 mL]) of water or orange juice, stir vigorously, and drink at once.
- Lymphangioleiomyomatosis:
- 2 mg by mouth once a day
- Note: Measure sirolimus whole blood trough concentrations in 10 - 20 days, with a dosage adjustment to maintain concentrations between 5 - 15 ng/mL
- Dosage adjustment may be based on simple proportion: New sirolimus dose = current dose x (target concentration divided by current concentration)
- Once maintenance dose is adjusted, continue on the new maintenance dose for at least 7 - 14 days before further dose adjustment
- Once a stable dose is achieved, perform therapeutic drug monitoring at least every 3 months
- Renal transplant:
- Initial Dose (Post Transplant):
- Give as soon as possible after transplantation, and 4 hours after cyclosporine
- Once maintenance dose is adjusted, continue on the new maintenance dose for ≥ 7 - 14 days before further dose adjustment
- Dosage adjustment may be based on simple proportion: New sirolimus dose = current dose x (target concentration/current concentration)
- Max Dose: 40 mg/day. If estimated dose is > 40 mg/day due to addition of loading dose (LD), administer LD over 2 days; monitor trough concentrations at least 3-4 days after LD(s)
- Low- to Moderate-Immunologic Risk:
- Give LD equivalent to 3X the maintenance dose.
- Give with cyclosporine and corticosteroids; progressively discontinue cyclosporine over 4-8 weeks at 2-4 months following transplantation. Adjust dose to maintain blood trough concentration with target-range
- Target sirolimus whole blood trough concentrations: 1st year: 16-24 ng/mL. Thereafter: 12-20 ng/mL
- High-Immunologic Risk:
- Give with cyclosporine and corticosteroids for the first 12 months following transplantation.
- Loading dose: Up to 15 mg on Day 1 post-transplantation.
- Maintenance: 5 mg/day beginning on day 2: obtain trough level between Days 5 and 7 and adjust daily dose thereafter
- Cyclosporine dosing: Up to 7 mg/kg/day in divided doses; subsequently adjust to achieve target whole blood trough concentrations
- Prednisone dosing: Minimum of 5 mg/day
- Antibody induction therapy may be used
- Low body weight (<40 kg):
- Adjust initial dose based on BSA to 1 mg/m2/day with a LD of 3 mg/m2
- Renal transplant, ≥13 years:
-
Initial Dose (Post-Transplant):
-
Give initial dose as soon as possible after transplantation, and 4 hours after cyclosporine
-
Once maintenance dose is adjusted, continue on the new maintenance dose for ≥ 7-14 days before further dose adjustment
-
Dosage adjustment may be based on simple proportion: New sirolimus dose = current dose x (target concentration/current concentration)
-
LD should be considered in addition to new maintenance dose when it is necessary to increase sirolimus trough concentrations: sirolimus LD = 3 x (new maintenance dose - current maintenance dose)
-
Max Dose: 40 mg/day. If estimated dose is >40 mg/day due to addition of LD, administer LD over 2 days; monitor trough concentrations at least 3-4 days after LD(s)
-
Low-Moderate Immunologic Risk:
-
Give LD equivalent to 3X the maintenance dose.
-
Give with cyclosporine and corticosteroids; progressively discontinue cyclosporine over 4-8 weeks at w2-4 months following transplantation. Adjust dose to maintain blood trough concentration within target range
-
Target sirolimus whole blood trough concentrations: 1st year: 16-24 ng/mL. Thereafter: 12-20 ng/mL
- Mild or moderate:
- Reduce maintenance dose by approximately 1/3
- Severe:
- Reduce maintenance dose by approximately 1/2
- Use is not recommended in liver or lung transplant patients - safety and efficacy have not been established. With liver transplantation - excess mortality, graft loss, and hepatic artery thrombosis (HAT) may result. With lung transplantation, bronchial anastomotic dehiscence may result
- Increased susceptibility to infection and the possible development of lymphoma and other malignancies may result from immunosuppression
- Hypersensitivity reactions
- Angioedema
- Fluid accumulation and wound healing
- Hyperlipidemia
- Renal function
- Proteinuria
- Latent viral infections
- Interstitial lung disease
- De Novo use without cyclosporine
- Increased risk of calcineurin inhibitor-induced HUS/TTP/TMA
- Increased risk for skin cancer
- Peripheral edema
- Hypertriglyceridemia
- Hypertension
- Hypercholesterolemia
- Creatinine increased
- Abdominal pain
- Diarrhea
- Headache
- Fever
- Urinary trace infection
- Anemia
- Nausea
- Arthralgia
- Pain
- Thrombocytopenia
- In general, the adverse effects are consistent with those listed in the adverse reactions section
- General supportive measures should be followed
- It is anticipated that sirolimus is not dialyzable to any significant extent
- Cyclosporine - concomitant use increases sirolimus concentrations; recommended to give sirolimus 4 hours after administration of cyclosporine oral solution and/or cyclosporine capsules. If cyclosporine is withdrawn from combination therapy, higher doses of sirolimus are needed to maintain the recommended sirolimus trough concentration ranges
- Strong CYP3A4/P-gp inducers or strong CYP3A4/P-gp inhibitors that decrease or increase sirolimus concentrations - avoid concomitant use
- Drugs that are known inhibitors/inducers of CYP3A4/P-gp - exercise caution when co-administered
- Grapefruit juice - inhibits the CYP3A4-mediated metabolism of sirolimus
- Vaccinations - nay be less effective during treatment. Avoid use of live vaccines (i.e., measles, mumps, rubella, oral polio, BCVG, yellow fever, varicella, TY21a typhoid)
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether the drug is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother
- Renal Impairment: None
- Hepatic Impairment: Reduce maintenance dose
- Pediatric Patients: The safety and efficacy in patients <13 years have not been established
- Geriatric Patients: Dose adjustments based upon age in geriatric renal patients are not necessary. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
- It is not known whether the drug is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
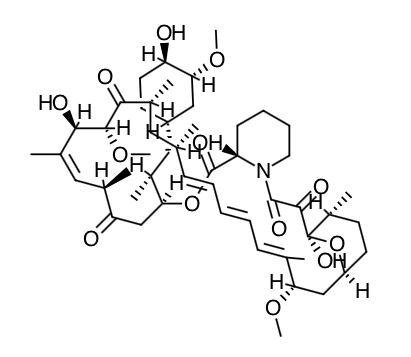
- Scientific Name: (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)- 9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-[(1R)-2- [(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy- 6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclohentriacontine- 1,5,11,28,29 (4H,6H,31H)-pentone
- Empirical Formula: C51H79NO13
- Molecular Weight: 914.2
- Sirolimus inhibits T-lymphocyte activation and proliferation that occurs in response to antigenic and cytokine (Interleukin [IL]-2, IL-4, and IL-15) stimulation by a mechanism that is distinct from that of other immunosuppressants. Sirolimus also inhibits antibody production. In cells, sirolimus binds to the immunophilin, FK Binding Protein-12 (FKBP-12), to generate an immunosuppressive complex. The sirolimus:FKBP-12 complex has no effect on calcineurin activity. This complex binds to and inhibits the activation of the mammalian Target Of Rapamycin (mTOR), a key regulatory kinase. This inhibition suppresses cytokine-driven T-cell proliferation, inhibiting the progression from the G1 to the S phase of the cell cycle.
- Studies in experimental models show that sirolimus prolongs allograft (kidney, heart, skin, islet, small bowel, pancreatico-duodenal, and bone marrow) survival in mice, rats, pigs, and/or primates. Sirolimus reverses acute rejection of heart and kidney allografts in rats and prolongs the graft survival in presensitized rats. In some studies, the immunosuppressive effect of sirolimus lasts up to 6 months after discontinuation of therapy. This tolerization effect is alloantigen-specific.
- In rodent models of autoimmune disease, sirolimus suppresses immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis
- Orally-administered sirolimus, at doses of 2 mg/day and 5 mg/day, significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at 6 months following transplantation compared with either azathioprine or placebo. There was no demonstrable efficacy advantage of a daily maintenance dose of 5 mg with a loading dose of 15 mg over a daily maintenance dose of 2 mg with a loading dose of 6 mg. Therapeutic drug monitoring should be used to maintain sirolimus drug levels within the target-range.
-
Absorption: Following administration of the oral solution, the mean times to peak concentration (Tmax) of sirolimus are approximately 1 hour and 2 hours in healthy subjects and renal transplant patients, respectively. The systemic availability of sirolimus is low, and was estimated to be approximately 14% after the administration of the oral solution. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of the oral solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2. Food Effects: To minimize variability in sirolimus concentrations, both the oral solution and tablets should be taken consistently with or without food. In healthy subjects, a high-fat meal (861.8 kcal, 54.9% kcal from fat) increased the mean total exposure (AUC) of sirolimus by 23 to 35%, compared with fasting. The effect of food on the mean sirolimus Cmax was inconsistent depending on the dosage form evaluated.
-
Distribution: The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins.
-
Metabolism: Sirolimus is a substrate for both CYP3A4 and P-gp. Sirolimus is extensively metabolized in the intestinal wall and liver and undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Inhibitors of CYP3A4 and P-gp increase sirolimus concentrations. Inducers of CYP3A4 and P-gp decrease sirolimus concentrations. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven (7) major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Sirolimus is the major component in human whole blood and contributes to more than 90% of the immunosuppressive activity.
-
Excretion: After a single dose of [14-C] sirolimus oral solution in healthy volunteers, the majority (91%) of radioactivity was recovered from the feces, and only a minor amount (2.2%) was excreted in urine. The mean ± SD terminal elimination half-life (t1/2) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours.
-
Specific Populations
-
Hepatic Impairment: Sirolimus was administered as a single, oral dose to subjects with normal hepatic function and to patients with Child-Pugh classification A (mild), B (moderate), or C (severe) hepatic impairment. Compared with the values in the normal hepatic function group, the patients with mild, moderate, and severe hepatic impairment had 43%, 94%, and 189% higher mean values for sirolimus AUC, respectively, with no statistically significant differences in mean Cmax As the severity of hepatic impairment increased, there were steady increases in mean sirolimus t1/2, and decreases in the mean sirolimus clearance normalized for body weight (CL/F/kg). The maintenance dose should be reduced by approximately one third in patients with mild-to-moderate hepatic impairment and by approximately one half in patients with severe hepatic impairment. It is not necessary to modify the loading dose in patients with mild, moderate, and severe hepatic impairment. Therapeutic drug monitoring is necessary in all patients with hepatic impairment.
-
Renal Impairment: The effect of renal impairment on the pharmacokinetics of sirolimus is not known. However, there is minimal (2.2%) renal excretion of the drug or its metabolites in healthy volunteers. The loading and the maintenance doses of Rapamune need not be adjusted in patients with renal impairment.
-
Pediatric: Sirolimus pharmacokinetic data were collected in concentration-controlled trials of pediatric renal transplant patients who were also receiving cyclosporine and corticosteroids. The target ranges for trough concentrations were either 10-20 ng/mL for the 21 children receiving tablets, or 5-15 ng/mL for the one child receiving oral solution. The children aged 6-11 years (n = 8) received mean ± SD doses of 1.75 ± 0.71 mg/day (0.064 ± 0.018 mg/kg, 1.65 ± 0.43 mg/m2). The children aged 12-18 years (n = 14) received mean ± SD doses of 2.79 ± 1.25 mg/day (0.053 ± 0.0150 mg/kg, 1.86 ± 0.61 mg/m2). At the time of sirolimus blood sampling for pharmacokinetic evaluation, the majority (80%) of these pediatric patients received the sirolimus dose at 16 hours after the once-daily cyclosporine dose.
-
Geriatric: Clinical studies of sirolimus did not include a sufficient number of patients > 65 years of age to determine whether they will respond differently than younger patients. After the administration of the oral solution or tablets, sirolimus trough concentration data in renal transplant patients > 65 years of age were similar to those in the adult population 18 to 65 years of age.
-
Gender: Sirolimus clearance in males was 12% lower than that in females; male subjects had a significantly longer t1/2 than did female subjects (72.3 hours versus 61.3 hours). Dose adjustments based on gender are not recommended.
-
Race: In the phase 3 trials using the solution or tablets and cyclosporine oral solution [MODIFIED] (e.g., Neoral Oral Solution) and/or cyclosporine capsules [MODIFIED] (e.g., Neoral Soft Gelatin Capsules), there were no significant differences in mean trough sirolimus concentrations over time between Black (n=190) and non-Black (n=852) patients during the first 6 months after transplantation.
-
Drug-Drug Interactions:
-
Sirolimus is known to be a substrate for both cytochrome CYP3A4 and P-gp. The pharmacokinetic interaction between sirolimus and concomitantly administered drugs is discussed below. Drug interaction studies have not been conducted with drugs other than those described below.
-
Cyclosporine:
-
Cyclosporine is a substrate and inhibitor of CYP3A4 and P-gp. Sirolimus should be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED). Sirolimus concentrations may decrease when cyclosporine is discontinued, unless the sirolimus dose is increased.
-
In a single-dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus tablets either simultaneously or 4 hours after a 300-mg dose of Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, mean Cmax and AUC were increased by 512% and 148%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after cyclosporine administration, sirolimus Cmax and AUC were both increased by only 33% compared with administration of sirolimus alone.
-
In a single dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus oral solution either simultaneously or 4 hours after a 300 mg dose of Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, the mean Cmax and AUC of sirolimus, following simultaneous administration were increased by 116% and 230%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were increased by only 37% and 80%, respectively, compared with administration of sirolimus alone.
-
In a single-dose cross-over drug-drug interaction study, 33 healthy volunteers received 5 mg the oral solution alone, 2 hours before, and 2 hours after a 300 mg dose of Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). When given 2 hours before Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were comparable to those with administration of sirolimus alone. However, when given 2 hours after, the mean Cmax and AUC of sirolimus were increased by 126% and 141%, respectively, relative to administration of sirolimus alone.
-
Mean cyclosporine Cmax and AUC were not significantly affected when the oral solution was given simultaneously or when administered 4 hours after Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). However, after multiple-dose administration of sirolimus given 4 hours after Neoral in renal post-transplant patients over 6 months, cyclosporine oral-dose clearance was reduced, and lower doses of Neoral Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) were needed to maintain target cyclosporine concentration.
-
In a multiple-dose study in 150 psoriasis patients, sirolimus 0.5, 1.5, and 3 mg/m/day was administered simultaneously with Sandimmune Oral Solution (cyclosporine Oral Solution) 1.25 mg/kg/day. The increase in average sirolimus trough concentrations ranged between 67% to 86% relative to when sirolimus was administered without cyclosporine. The intersubject variability (% CV) for sirolimus trough concentrations ranged from 39.7% to 68.7%. There was no significant effect of multiple-dose sirolimus on cyclosporine trough concentrations following Sandimmune Oral Solution (cyclosporine oral solution) administration. However, the % CV was higher (range 85.9% - 165%) than those from previous studies.
-
Diltiazem: Diltiazem is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 10 mg of sirolimus oral solution and 120 mg of diltiazem to 18 healthy volunteers significantly affected the bioavailability of sirolimus. Sirolimus Cmax, Tmax, and AUC were increased 1.4-, 1.3-, and 1.6-fold, respectively. Sirolimus did not affect the pharmacokinetics of either diltiazem or its metabolites desacetyldiltiazem and desmethyldiltiazem.
-
Erythromycin: Erythromycin is a substrate and inhibitor of CYP3A4 and P-gp; co- administration of sirolimus oral solution or tablets and erythromycin is not recommended. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 800 mg q 8h of erythromycin as erythromycin ethylsuccinate tablets at steady state to 24 healthy volunteers significantly affected the bioavailability of sirolimus and erythromycin. Sirolimus Cmax and AUC were increased 4.4- and 4.2-fold respectively and Tmax was increased by 0.4 hr. Erythromycin Cmax and AUC were increased 1.6- and 1.7-fold, respectively, and Tmax was increased by 0.3 hr.
-
Ketoconazole: Ketoconazole is a strong inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and ketoconazole is not recommended. Multiple-dose ketoconazole administration significantly affected the rate and extent of absorption and sirolimus exposure after administration of the oral solution, as reflected by increases in sirolimus Cmax, Tmax, , and AUC of 4.3-fold, 38%, and 10.9-fold, respectively. However, the terminal of sirolimus was not changed. Single-dose sirolimus did not affect steady-state 12-hour plasma ketoconazole concentrations.
-
Rifampin: Rifampin is a strong inducer of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and rifampin is not recommended. In patients where rifampin is indicated, alternative therapeutic agents with less enzyme induction potential should be considered. Pretreatment of 14 healthy volunteers with multiple doses of rifampin, 600 mg daily for 14 days, followed by a single 20-mg dose of sirolimus oral solution, greatly decreased sirolimus AUC and Cmax by about 82% and 71%, respectively.
-
Verapamil: Verapamil is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 180 mg q 12h of verapamil at steady state to 26 healthy volunteers significantly affected the bioavailability of sirolimus and verapamil. Sirolimus Cmax and AUC were increased 2.3- and 2.2-fold, respectively, without substantial change in Tmax. The Cmax and AUC of the pharmacologically active S(-) enantiomer of verapamil were both increased 1.5-fold and Tmax was decreased by 1.2 hr.
-
Drugs which may be co-administered without dose adjustment: Clinically significant pharmacokinetic drug-drug interactions were not observed in studies of acyclovir, atorvastatin, digoxin, glyburide, nifedipine, norgestrel/ethinyl estradiol (Lo/Ovral) , prednisolone, and sulfamethoxazole/trimethoprim (Bactrim); sirolimus and these drugs may be co-administered without dose adjustments.
-
Other drug-drug interactions: Co-administration of sirolimus with other known strong inhibitors of CYP3A4 and/or P-gp (such as voriconazole, itraconazole, telithromycin, or clarithromycin) or other known strong inducers of CYP3A4 and/or P-gp (such as rifabutin) is not recommended. In patients in whom strong inhibitors or inducers of CYP3A4 are indicated, alternative therapeutic agents with less potential for inhibition or induction of CYP3A4 should be considered. Care should be exercised when drugs or other substances that are substrates and/or inhibitors or inducers of CYP3A4 are administered concomitantly with sirolimus. Other drugs that have the potential to increase sirolimus blood concentrations include (but are not limited to): calcium channel blockers (nicardipine), antifungal agents (clotrimazole, fluconazole), antibiotics (troleandomycin), gastrointestinal prokinetic agents (cisapride, metoclopramide), others drugs (bromocriptine, cimetidine, danazol, HIV-protease inhibitors (e.g., ritonavir, indinavir). Other drugs that have the potential to decrease sirolimus concentrations include (but are not limited to): anticonvulsants (carbamazepine, phenobarbital, phenytoin), antibiotics (rifapentine).
-
Other drug-food interactions: Grapefruit juice reduces CYP3A4-mediated drug metabolism. Grapefruit juice must not be taken with or used for dilution of sirolimus.
-
Drug-herb interactions: St. John's Wort (hypericum perforatum) induces CYP3A4 and P-gp. Since sirolimus is a substrate for both cytochrome CYP3A4 and P-gp, there is the potential that the use of St. John's Wort in patients receiving sirolimus could result in reduced sirolimus concentrations.
- Advise patients that exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor because of the increased risk for skin cancer.
- Advise women of childbearing potential of the potential risks during pregnancy and told that they should use effective contraception prior to initiation of therapy, during therapy, and for 12 weeks after therapy has been stopped.
Indications
Dosing (Adult)
Dosing (Pediatric)
Hepatic Dosing
Black Box Warnings
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

