Rivastigmine (Exelon): Drug Monograph
|
|---|
- Treatment of mild to moderate dementia of the Alzheimer's type
- Treatment of mild to moderate dementia associated with Parkinson's disease
- Treatment of severe dementia of the Alzheimer's type (Transdermal Patch only)
-
General Dosing & Administration Notes:
- Take with meals in divided doses in the morning and evening.
- Solution and capsules may be interchanged at equal doses.
- Solution: Dose may be swallowed directly from the syringe or first mixed with a small glass of water, cold fruit juice, or soda. Patients should stir and drink the mixture.
- Patch: Do not use if the pouch seal is broken or the patch is cut damaged or changed in any way. Apply once a day; press down firmly for 30 seconds until the edges stick well when applying to clean, dry, hairless, intact, healthy skin in a place that will not be rubbed against by tight clothing. Use the upper or lower back as the site of application. Replace with a new patch every 24 hours. Change application site daily; do not apply a new patch to the same location for at least 14 days.
- Alzheimer's disease, mild to moderate dementia:
- Capsule/solution:
- 1.5 mg by mouth twice daily
- May increase dose to 3 mg twice daily after at least 2 weeks; subsequent increases to 4.5 mg twice daily and 6 mg twice daily should be attempted after a minimum of 2 weeks at the previous dose
- Usual: 6-12 mg/day (3-6 mg twice daily)
- Maximum: 12 mg/day (6 mg twice daily)
- Patch:
- Apply one 4.6 mg/24 hours patch daily.
- Increase dose only after a minimum of 4 weeks at the previous dose
- Maintenance: 9.5 mg/24 hours daily or 13.3 mg/24 hours daily
- Maximum 13.3 mg/24 hours
- Alzheimer's disease, severe dementia:
- Patch:
- Apply one 4.6 mg/24 hours patch daily
- Increase dose only after a minimum of 4 weeks at the previous dose
- Maintenance: 13.3 mg/24 hours daily
- Maximum: 13.3 mg/24 hours
- Parkinson's disease, mild to moderate dementia:
- Capsule/solution:
- 1.5 mg by mouth twice daily
- May increase to 3 mg twice daily after at least 4 weeks; subsequent increases to 4.5 mg twice daily and 6 mg twice daily should be attempted after a minimum of 4 weeks at the previous dose
- Maximum: 12 mg/day (6 mg twice daily)
- Patch:
- Apply one 4.6 mg/24 hours patch daily
- Increase dose only after a minimum of 4 weeks at the previous dose
- Maintenance: 9.5 mg/24 hours daily or 13.3 mg/24 hours daily
- Maximum: 13.3 mg/24 hours
- Conversions, switching to patch from capsules/solution:
- Total daily oral dose <6 mg = 4.6 mg/24 hours patch (applied just following the last oral dose)
- Total daily oral dose 6-12 mg = 9.5 mg/24 hours patch (applied just following the last oral dose)
- Mild to moderate (Child-Pugh score 5-9):
- Capsules/solution: May be able to only tolerate lower doses
- Patch: Consider using the 4.6 mg/24 hours patch as both initial and maintenance dose
- Capsules: 1.5 mg, 3 mg, 4.5 mg, 6 mg
- Solution: 2 mg/mL [120 mL]
- Patch: 4.6 mg/24 hrs, 9,5 mg/24 hrs, 13.3 mg/24 hrs [30S]
- Known hypersensitivity to rivastigmine, other carbamate derivatives or other components of the formulation
- History of application site reaction with rivastigmine transdermal patch suggestive of allergic contact dermatitis, in the absence of negative allergy testing
- Gastrointestinal adverse reactions - may include significant nausea, vomiting, diarrhea, anorexia/decreased appetite, and weight loss, and may necessitate treatment interruption. Dehydration may result from prolonged vomiting or diarrhea and can be associated with serious outcomes.
- Disseminated allergic dermatitis - may occur after oral or transdermal administration. Discontinue use. In patients with suspected allergic contact dermatitis after transdermal rivastigmine use, switch to oral drug only after negative allergy testing.
- Neurologic effects - extrapyramidal symptoms may be exacerbated or induced (e.g., worsening of parkinsonian symptoms, particularly tremor)
- Peptic ulcers/gastrointestinal bleeding - may increase gastric acid secretion due to increased cholinergic activity. Monitor those patients at increased risk for developing ulcers.
- Use with anesthesia - likely to exaggerate succinylcholine-type muscle relaxation during anesthesia
- Cardiac conduction effects - may have vagotonic effects on heart rate (e.g., bradycardia). The potential for action may be particularly important in patients with sick sinus syndrome ore other supraventricular cardiac conduction conditions.
- Genitourinary effects - may cause urinary obstruction
- Pulmonary effects - use with care in patients with a history of asthma or obstructive pulmonary disease
- Impairment in driving or use of machinery - may result in adverse reactions that are detrimental to these functions. Routinely evaluate the patient's ability to continue driving or operating machinery.
- No further dose should be administered for the next 24 hours in cases of asymptomatic overdoses due to the short plasma half-life of about 1 hour and a moderate duration of acetylcholinesterase inhibition of 8 to 10 hours.
- General supportive measures should be utilized.
- Overdosage can result in cholinergic crisis characterized by severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved.
- Additional symptoms associated with overdose are diarrhea, abdominal pain, dizziness, tremor, headache, somnolence, confusional state, hyperhidrosis, hypertension, hallucinations and malaise.
- Dialysis (hemodialysis, peritoneal dialysis, or hemofiltration) would not be clinically indicated due to the short half-life of rivastigmine.
- If severe nausea and vomiting are present, the use of antiemetics should be considered.
- A fatal outcome has been rarely reported with rivastigmine.
- Metoclopramide beta-blockers, or cholinomimetic and anticholinergic drugs - concomitant use is not recommended.
- Pregnancy: Pregnancy Category B
- Labor and Delivery: None
- Nursing Mothers: It is not known whether rivastigmine is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
- Renal Impairment: Patients with moderate to severe impairment may be able to only tolerate lower doses.
- Hepatic Impairment: Patients with mild (Child-Pugh score 5 to 6) and moderate (Child-Pugh score 7 to 9) impairment may be able to only tolerate lower doses. No data are available on the use of rivastigmine in patients with severe impairment.
- Pediatric Patients: Safety and effectiveness have not been established. The use in pediatric patients (below 18 years of age) is not recommended.
- Geriatric Patients: No overall differences observed between elderly patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out
- Low Body Weight: Carefully titrate and monitor patients with low body weight (less than 50 kg) for toxicities (e.g., excessive nausea, vomiting) and consider reducing the dose if such toxicities develop.
- It is not known whether rivastigmine is excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
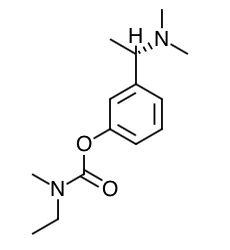
- Scientific Name: (S)-N-Ethyl-N- methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate hydrogen-(2R,3R)-tartrate
- Empirical Formula: C14H22N2O2.C4H6O6
- Molecular Weight: 400.43
- Although the precise mechanism of action of rivastigmine is unknown, it is thought to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. Therefore, the effect of rivastigmine may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that rivastigmine alters the course of the underlying dementing process.
- After a 6-mg dose of rivastigmine, anticholinesterase activity is present in cerebrospinal fluid (CSF) for about 10 hours, with a maximum inhibition of about 60% 5 hours after dosing.
- In vitro and in vivo studies demonstrate that the inhibition of cholinesterase by rivastigmine is not affected by the concomitant administration of memantine, an N-methyl-D-aspartate receptor antagonist.
- Rivastigmine shows linear pharmacokinetics up to 3 mg twice a day but is nonlinear at higher doses. Doubling the dose from 3 mg to 6 mg twice a day results in a 3-fold increase in AUC. The elimination half-life is about 1.5 hours, with most elimination as metabolites via the urine.
- Absorption: Rivastigmine is rapidly and completely absorbed. Peak plasma concentrations are reached in approximately 1 hour. Absolute bioavailability after a 3-mg dose is about 36%. Administration of rivastigmine with food delays absorption (Tmax) by 90 minutes lowers Cmax by approximately 30% and increases AUC by approximately 30%.
- Distribution: Rivastigmine is weakly bound to plasma proteins (approximately 40%) over the therapeutic range. It readily crosses the blood-brain barrier, reaching CSF peak concentrations in 1.4 to 2.6 hours. It has an apparent volume of distribution in the range of 1.8 to 2.7 L/kg.
- Metabolism: Rivastigmine is rapidly and extensively metabolized, primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite. Based on evidence from in vitro and animal studies, the major cytochrome P450 isozymes are minimally involved in rivastigmine metabolism. Consistent with these observations is the finding that no drug interactions related to cytochrome P450 have been observed in humans.
- Elimination: The major pathway of elimination is via the kidneys. Following administration of 14C-rivastigmine to 6 healthy volunteers, total recovery of radioactivity over 120 hours was 97% in urine and 0.4% in feces. No parent drug was detected in urine. The sulfate conjugate of the decarbamylated metabolite is the major component excreted in urine and represents 40% of the dose. Mean oral clearance of rivastigmine is 1.8 ± 0.6 L/min after 6 mg twice a day.
- Age: Following a single 2.5-mg oral dose to elderly volunteers (60 years and older, n=24) and younger volunteers (n=24), mean oral clearance of rivastigmine was 30% lower in elderly (7 L/min) than in younger subjects (10 L/min).
- Gender and Race: Population pharmacokinetic analysis of oral rivastigmine indicated that neither gender (n=277 males and 348 females) nor race (n=575 Caucasian, 34 Black, 4 Asian, and 12 Other) affected clearance of the drug.
- Body Weight: A relationship between drug exposure at steady-state (rivastigmine and metabolite NAP226-90) and body weight was observed in Alzheimer's dementia patients. Rivastigmine exposure is higher in subjects with low body weight. Compared to a patient with a body weight of 65 kg, the rivastigmine steady-state concentrations in a patient with a body weight of 35 kg would be approximately doubled, while for a patient with a body weight of 100 kg the concentrations would be approximately halved.
- Renal Impairment: Following a single 3-mg dose, mean oral clearance of rivastigmine is 64% lower in moderately impaired renal patients (n=8, GFR=10 to 50 mL/min) than in healthy subjects (n=10, GFR ≥60 mL/min); Cl/F=1.7 L/min and 4.8 L/min, respectively. In patients with severe renal impairment (n=8, GFR <10 mL/min), mean oral clearance of rivastigmine is 43% higher than in healthy subjects (n=10, GFR ≥60 mL/min); Cl/F=6.9 L/min and 4.8 L/min, respectively. For unexplained reasons, the severely impaired renal patients had a higher clearance of rivastigmine than moderately impaired patients.
- Hepatic Impairment: Following a single 3-mg dose, mean oral clearance of rivastigmine was 60% lower in hepatically impaired patients (n=10, biopsy proven) than in healthy subjects (n=10). After multiple 6-mg twice a day oral dosing, the mean clearance of rivastigmine was 65% lower in mild (n=7, Child-Pugh score 5 to 6) and moderate (n=3, Child-Pugh score 7 to 9) hepatically impaired patients (biopsy proven, liver cirrhosis) than in healthy subjects (n=10).
- Smoking: Following oral rivastigmine administration (up to 12 mg/day) with nicotine use, population pharmacokinetic analysis showed increased oral clearance of rivastigmine by 23% (n=75 smokers and 549 nonsmokers).
- Drug Interaction Studies
- Effect of Rivastigmine on the Metabolism of Other Drugs: Rivastigmine is primarily metabolized through hydrolysis by esterases. Minimal metabolism occurs via the major cytochrome P450 isoenzymes. Based on in vitro studies, no pharmacokinetic drug interactions with drugs metabolized by the following isoenzyme systems are expected: CYP1A2, CYP2D6, CYP3A4/5, CYP2E1, CYP2C9, CYP2C8, CYP2C19, or CYP2B6. No pharmacokinetic interaction was observed between rivastigmine taken orally and digoxin, warfarin, diazepam or fluoxetine in studies in healthy volunteers. The increase in prothrombin time induced by warfarin is not affected by administration of rivastigmine.
- Effect of Other Drugs on the Metabolism of Rivastigmine: Drugs that induce or inhibit CYP450 metabolism are not expected to alter the metabolism of rivastigmine. Population pharmacokinetic analysis with a database of 625 patients showed that the pharmacokinetics of rivastigmine taken orally were not influenced by commonly prescribed medications such as antacids (n=77), antihypertensives (n=72), beta-blockers (n=42), calcium channel blockers (n=75), antidiabetics (n=21), NSAIDs (n=79), estrogens (n=70), salicylate analgesics (n=177), antianginals (n=35) and antihistamines (n=15).
- Caregivers should be advised of the high incidence of nausea and vomiting associated with the use of the drug along with the possibility of anorexia and weight loss. Caregivers should be encouraged to monitor for these adverse events and inform the physician if they occur.
- It is critical to inform caregivers that if therapy has been interrupted for more than several days, the next dose should not be administered until they have discussed this with the physician.
- Caregivers and patients should be advised that allergic skin reactions have been reported in association with rivastigmine regardless of formulation (capsules, oral solution or transdermal patch). In case of skin reaction, patients should consult with their physician immediately.
- Caregivers should be instructed in the correct procedure for administering the oral solution. In addition, they should be informed of the existence of an Instruction Sheet (included with the product) describing how the solution is to be administered. They should be urged to read this sheet prior to administering the oral solution.
- Caregivers should direct questions about the administration of the solution to either their physician or pharmacist.
- Caregivers and patients should be advised that colinomimetics, including rivastigmine, may exacerbate or induce extrapyramidal symptoms. Worsening in patients with Parkinson's disease, including an increased incidence or intensity or tremor, has been observed.
- Instruct patients to rotate application site of the patch, not to use the same site within 14 days, to replace the patch every 24 hours at consistent time of day, and to wear only 1 patch at a time.
- Instruct patient to avoid exposure to external heat for long periods.
- Instruct on proper usage and discarding of patch.
- In case of accidental contact with eyes or if eyes become red after handling the patch, instruct the patient to rinse immediately with plenty of water and seek medical advice if symptoms do not resolve.
- Advise patients not to take rivastigmine capsules or oral solution, or other drugs with cholinergic effects while wearing the patch.
- Instruct the patient to inform their physician if application-site reactions spread beyond the patch size, if there is evidence of more intense local reaction and if symptoms do not significantly improve within 49 hours after patch removal.
Indications
Dosing (Adult)
Hepatic Dosing
Dosage Forms
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|