Risperidone (Risperdal)
|
|---|
- Treatment of schizophrenia in adults an adolescents aged 13-17 years
- Treatment of acute manic or mixed episodes associated with bipolar I disorder as monotherapy in adults and pediatrics (10-17 years of age) or as adjunctive therapy with lithium or valproate in adults
- Treatment of irritability associated with autistic disorder (e.g., symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods) in children and adolescents aged 5-17 years
- General Dosing & Administration Notes:
- Oral solution may be mixed with a beverage prior to administration, and is compatible in the following beverages: water, coffee, orange juice, and low-fat milk; not compatible with cola or tea.
- Orally disintegrating tablets should be consumed immediately, as the tablet cannot be stored once removed from the blister unit.
- Swallow with or without liquid.
- Do not attempt to split or to chew the tablet.
- Bipolar mania:
- 2 - 3 mg by mouth once a day;
- May increase at intervals of ≥ 24 hours, by 1 mg daily
- Target: 1 - 6 mg/day
- Maximum: 6 mg/day
- If experiencing persistent somnolence may administer 1/2 daily dose twice daily
- Schizophrenia:
- May be administered once or twice daily
- 2 mg by mouth once a day or in two divided doses per day
- May increase at intervals of ≥ 24 hours, by 1 - 2 mg daily
- Target: 4 - 8 mg/day; efficacy demonstrated in a range of 4 - 16 mg/day
- Maintenance: 2 - 8 mg/day
- Maximum: 16 mg/day
- If experiencing persistent somnolence, may administer 1/2 daily dose twice daily
- Schizophrenia, 13-17 years:
- 0.5 mg/day in morning or evening
- May increase at intervals of ≥24 hours, by 0.5-1mg daily
- Target: 3 mg/day; efficacy demonstrated in a range of 1-6 mg/day
- Maximum: 6 mg/day
- If experiencing persistent somnolence, may administer 1/2 daily dose twice daily
- Bipolar mania, 10-17 years:
- 0.5 mg/day in morning or evening
- May increase at intervals of ≥24 hours, by 0.5-1mg daily
- Target: 1-2.5 mg/day; efficacy demonstrated in range of 0.5-6 mg
- Maximum: 6 mg/day
- If experiencing persistent somnolence, may administer 1/2 daily dose twice daily
- Autistic disorder, 5-17 years:
- Total daily dose may be administered once daily or divided and given twice daily
- <20 kg: 0.25 mg/day
- ≥20 kg: 0.5 mg/day
- May increase after a minimum of 4 days
- Target, <20 kg: 0.5 mg/day
- Target, ≥20 kg: 1 mg/day
- Maintain target dose for a minimum of 14 days. If not achieving sufficient response, may increase at intervals of ≥2 weeks, by 0.25 mg/day (<20 kg) or 0.5 mg/day (≥20 kg)
- Range: 0.5-3 mg/day
- Once sufficient response is achieved and maintained, consider gradually lowering the dose
- If experiencing persistent somnolence, may give daily dose at bedtime, divide daily dose to twice daily, or reduce dose
- Severe, CrCl <30 mL/min:
- 0.5 mg twice daily
- May increase by ≤0.5 mg, administered twice daily. For doses >1.5 mg twice daily, increase in intervals of ≥1 week
- 10-15 points on Child-Pugh system:
- 0.5 mg twice daily
- May increase by ≤0.5 mg, administered twice daily. For doses >1.5 mg twice daily, increase in intervals of ≥1 week
- Tablets: 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg
- Oral solution: 1 mg/mL
- Orally disintegrating tablets: 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg
- Elderly patients with dementia-related psychosis are at an increased risk of death. Most of the deaths appear to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature.
- Risperidone is not approved for the treatment of patients with dementia-related psychosis.
- Cerebrovascular events, including stroke, in elderly patients with dementia-related psychosis - not approved for use in these patients
- Neuroleptic malignant syndrome
- Tardive dyskinesia
- Hyperglycemia and diabetes mellitus
- Hyperprolactinemia
- Orthostatic hypotension
- Leukopenia, neutropenia, and agranulocytosis - patients with a history of a clinically significant low white blood cell count or a drug-induced leukopenia/neutropenia should have their complete blood count monitored frequently during the first few months of therapy ad discontinuation of risperidone should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors
- Potential for cognitive and motor impairment
- Seizures
- Dysphagia
- Priapism
- Disruption of body temperature regulation
- Antiemetic effect
- Suicide
- Increased sensitivity in patients with Parkinson's disease or those with dementia with Lewy Bodies
- Diseases or conditions that could affect metabolism or hemodynamic responses
- Somnolence
- Appetite increased
- Fatigue
- Rhinitis
- Upper respiratory tract infection
- Vomiting
- Coughing
- Urinary incontinence
- Saliva increased
- Constipation
- Fever
- Parkinsonism
- Dystonia
- Abdominal pain
- Anxiety
- Nausea
- Dizziness
- Dry mouth
- Tremor
- Rash
- Akathisia
- Dyspepsia
- Reactions associated with discontinuation:
- Somnolence
- Nausea
- Abdominal pain
- Dizziness
- Vomiting
- Agitation
- Akathisia
- Reported signs and symptoms are those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. Other signs may include hyponatremia, hypokalemia, prolonged QT, widened QRS, and seizure.
- In case of acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation.
- Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered.
- In patients that ingested the orally disintegrating tablets, pill fragments may not appear in gastric contents obtained with lavage due to the rapid disintegration of the tablets.
- The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
- Commence cardiovascular monitoring immediately.
- No specific antidote to risperidone. Appropriate supportive measures should be instituted and the possibility of multiple drug involvement should be considered.
- Other centrally acting drugs - use caution when administering due to CNS effects. Avoid alcohol.
- Due to hypotensive effects, hypotensive effects of other drugs with this potential may be enhanced.
- Levodopa and dopamine -agonists effects may be antagonized
- Cimetidine and ranitidine - increase the bioavailability of risperidone
- Clozapine - may decrease clearance of risperidone
- Fluoxetine and paroxetine - increase plasma concentrations of risperidone
- Carbamazepine and other enzyme inducers - decrease plasma concentrations of risperidone
- Pregnancy: Pregnancy Category C
- Labor and Delivery: The effect in humans is unknown.
- Nursing Mothers: Risperidone and 9-hydroxyrisperidone are excreted in human breast milk. Women receiving risperidone should not breast-feed.
- Renal Impairment: Doses should be reduced in patients with renal disease (moderate to severe).
- Hepatic Impairment: Doses should be reduced in patients with liver disease.
- Pediatric Patients: Safety and effectiveness have not been established for schizophrenia less than 13 years of age, for bipolar mania less than 10 years of age, and for autistic disorder less than 5 years of age.
- Geriatric Patients: Predisposition to hypotension or for whom hypotension poses a risk: lower initial dose (0.5 mg twice daily), followed by increases in dose in increments of no more than 0.5 mg twice daily. Increases to dosages above 1.5 mg twice daily should occur at intervals of at least 1 week. Monitoring of orthostatic vital signs should be considered in patients for whom this is of concern. May also be useful to monitor renal function. Risperidone is not approved for the treatment of patients with dementia-related psychosis.
- Risperidone and 9-hydroxyrisperidone are excreted in human breast milk. Women receiving risperidone should not breast-feed.
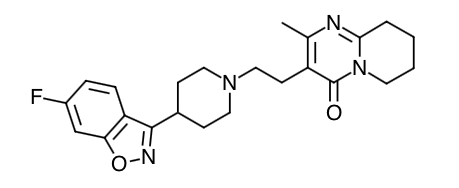
- Scientific Name: 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl) 1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- Empirical Formula: C23H27FN4O2
- Molecular Weight: 410.49
- The mechanism of action of risperidone, as with other drugs used to treat schizophrenia, is unknown. However, it has been proposed that the drug's therapeutic activity in schizophrenia is mediated through a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2) receptor antagonism.
- Risperidone is a selective monoaminergic antagonist with high affinity (Ki of 0.12 to 7.3 nM) for the serotonin Type 2 (5HT2), dopamine Type 2 (D2), α1 and α2 adrenergic, and H1 histaminergic receptors. Risperidone acts as an antagonist at other receptors, but with lower potency. Risperidone has low to moderate affinity (Ki of 47 to 253 nM) for the serotonin 5HT1C, 5HT1D, and 5HT1A receptors, weak affinity (Ki of 620 to 800 nM) for the dopamine D1 and haloperidol-sensitive sigma site, and no affinity (when tested at concentrations >10-5M) for cholinergic muscarinic or β1 and β2 adrenergic receptors.
- The clinical effect from risperidone results from the combined concentrations of risperidone and its major metabolite, 9-hydroxyrisperidone. Antagonism at receptors other than D2 and 5HT2 may explain some of the other effects of risperidone.
- Absorption: Risperidone is well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. Pharmacokinetic studies showed that risperidone orally disintegrating tablets and the oral solution are bioequivalent to the tablets. Plasma concentrations of risperidone, its major metabolite, 9-hydroxyrisperidone, and risperidone plus 9-hydroxyrisperidone are dose proportional over the dosing range of 1 to 16 mg daily (0.5 to 8 mg twice daily). Following oral administration of solution or tablet, mean peak plasma concentrations of risperidone occurred at about 1hour. Peak concentrations of 9-hydroxyrisperidone occurred at about 3 hours in extensive metabolizers, and 17 hours in poor metabolizers. Steady-state concentrations of risperidone are reached in 1 day in extensive metabolizers and would be expected to reach steady state in about 5 days in poor metabolizers. Steady-state concentrations of 9-hydroxyrisperidone are reached in 5-6 days (measured in extensive metabolizers). Food does not affect either the rate or extent of absorption of risperidone. Thus, risperidone can be given with or without meals.
- Distribution: Risperidone is rapidly distributed. The volume of distribution is 1-2 L/kg. In plasma, risperidone is bound to albumin and α1-acid glycoprotein. The plasma protein binding of risperidone is 90%, and that of its major metabolite, 9-hydroxyrisperidone, is 77%. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites. High therapeutic concentrations of sulfamethazine (100 mcg/mL), warfarin (10 mcg/mL), and carbamazepine (10mcg/mL) caused only a slight increase in the free fraction of risperidone at 10 ng/mL and 9-hydroxyrisperidone at 50 ng/mL, changes of unknown clinical significance.
- Metabolism: Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme, CYP 2D6. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone. CYP 2D6, also called debrisoquin hydroxylase, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP 2D6 is subject to genetic polymorphism (about 6%-8% of Caucasians, and a very low percentage of Asians, have little or no activity and are "poor metabolizers") and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP 2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP 2D6 metabolizers convert it much more slowly. Although extensive metabolizers have lower risperidone and higher 9-hydroxyrisperidone concentrations than poor metabolizers, the pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, are similar in extensive and poor metabolizers. Risperidone could be subject to two kinds of drug-drug interactions. First, inhibitors of CYP 2D6 interfere with conversion of risperidone to 9-hydroxyrisperidone. This occurs with quinidine, giving essentially all recipients a risperidone pharmacokinetic profile typical of poor metabolizers. The therapeutic benefits and adverse effects of risperidone in patients receiving quinidine have not been evaluated, but observations in a modest number (n≅70) of poor metabolizers given risperidone do not suggest important differences between poor and extensive metabolizers. Second, co-administration of known enzyme inducers (e.g., carbamazepine, phenytoin, rifampin, and phenobarbital) with risperidone may cause a decrease in the combined plasma concentrations of risperidone and 9-hydroxyrisperidone. It would also be possible for risperidone to interfere with metabolism of other drugs metabolized by CYP 2D6. Relatively weak binding of risperidone to the enzyme suggests this is unlikely.
- Elimination: Risperidone and its metabolites are eliminated via the urine and, to a much lesser extent, via the feces. As illustrated by a mass balance study of a single 1 mg oral dose of 14C-risperidone administered as solution to three healthy male volunteers, total recovery of radioactivity at 1 week was 84%, including 70% in the urine and 14% in the feces. The apparent half-life of risperidone was 3 hours (CV=30%) in extensive metabolizers and 20 hours (CV=40%) in poor metabolizers. The apparent half-life of 9-hydroxyrisperidone was about 21 hours (CV=20%) in extensive metabolizers and 30 hours (CV=25%) in poor metabolizers. The pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, were similar in extensive and poor metabolizers, with an overall mean elimination half-life of about 20 hours.
- Special Populations
- Renal Impairment: In patients with moderate to severe renal disease, clearance of the sum of risperidone and its active metabolite decreased by 60% compared to young healthy subjects. Risperidone doses should be reduced in patients with renal disease.
- Hepatic Impairment: While the pharmacokinetics of risperidone in subjects with liver disease were comparable to those in young healthy subjects, the mean free fraction of risperidone in plasma was increased by about 35% because of the diminished concentration of both albumin and α1acid glycoprotein. Risperidone doses should be reduced in patients with liver disease.
- Elderly: In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives were prolonged compared to young healthy subjects. Dosing should be modified accordingly in the elderly patients.
- Pediatric: The pharmacokinetics of risperidone and 9-hydroxyrisperidone in children were similar to those in adults after correcting for the difference in body weight.
- Race and Gender Effects: No specific pharmacokinetic study was conducted to investigate race and gender effects, but a population pharmacokinetic analysis did not identify important differences in the disposition of risperidone due to gender (whether corrected for body weight or not) or race.
- Patients should be advised of the risk of orthostatic hypotension, especially during the period of initial dose titration.
- Since risperidone has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy does not affect them adversely.
- Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy.
- Patients should be advised not to breast-feed an infant if they are taking risperidone.
- Patients should be advised to inform their physician if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
- Patients should be advised to avoid alcohol while taking risperidone.
- Phenylalanine is a component of aspartame. Each 4 mg risperidone orally disintegrating tablet contains 0.84 phenylalanine; each 3 mg tablet contains 0.63 phenylalanine; each 2 mg tablet contains 0.42 phenylalanine; each 1 mg tablet contains 0.28 mg phenylalanine; and each 0.5 mg tablet contains 0.14 mg phenylalanine.
Indications
Dosing (Adult)
Dosing (Pediatric)
Renal Dosing
Hepatic Dosing
Dosage Forms
Black Box Warnings
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





