Rasagiline (Azilect): Drug Monograph
|
|---|
- General Notes:
- May be administered with or without food
- Parkinson's disease:
- Monotherapy of adjunctive therapy in patients not taking levodopa:
- 1 mg once daily
- Patients taking levodopa with or without other Parkinson's disease drugs:
- 0.5 mg once daily. May increase to 1 mg once daily if a sufficient clinical response is not achieved.
- May consider dose reduction of concomitant levodopa based upon individual response
- Ciprofloxacin or other CYP1A2 inhibitors:
- Administer rasagiline at 0.5 mg once daily
- Mild impairment, Child-Pugh Class 5-6:
- 0.5 mg once daily
- Moderate or severe impairment, Child- Pugh Class 7-15:
- Do not use
- Concomitant use of meperidine, tramadol, methadone, propoxyphene, dextromethorphan, St. John's Wort, cyclobenzaprine or another (selective or non-selective) MAO inhibitor
- May cause hypertension (including severe hypertensive syndromes) at recommended doses
- May cause serotonin syndrome when used with antidepressants
- May cause falling asleep during activities of daily living, daytime drowsiness, and somnolence
- May cause hypotension, especially orthostatic
- May cause or exacerbate dyskinesia. Decreasing the levodopa dose may lessen or eliminate this side effect
- May cause hallucinations and psychotic-like behavior
- May cause impulse control/compulsive behaviors
- May cause withdrawal-emergent hyperpyrexia and confusion
- Increased risk of melanoma; monitor patients for melanoma on a regular basis
- Monotherapy:
- Flu syndrome
- Arthralgia
- Depression
- Dyspepsia
- Adjunct without levodopa:
- Peripheral edema
- Fall
- Arthralgia
- Cough
- Insomnia
- Adjunct to levodopa:
- Dyskinesia
- Accidental injury
- Weight loss
- Postural hypotension
- Vomiting
- Anorexia
- Arthralgia
- Abdominal pain
- Nausea
- Constipation
- Dry mouth
- Rash
- Abnormal dreams
- Fall
- Tenosynovitis
- Signs and symptoms may not appear immediately - may have delays of up to 12 hours after ingestion of drug and the appearance of signs.
- Signs and symptoms may include: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
- Treatment should be symptomatic and supportive. Support respiration; monitor body temperature; intensive management of hyperpyrexia may be required; maintenance of fluid and electrolyte balance is essential.
- Meperidine - risk of serotonin syndrome
- Dextromethorphan - risk of psychosis or bizarre behavior
- MAO inhibitors - risk of non-selective MAO inhibition and hypertensive crisis
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether this drug is excreted in human milk. Caution should be exercised when administered to a nursing woman.
- Renal Impairment: None
- Hepatic Impairment: Dosage adjustment for mild impairment; do not use in moderate or severe impairment.
- Pediatric Patients: The safety and effectiveness have not been established.
- Geriatric Patients: No significant differences in the safety profile of geriatric and non-geriatric patients.
- It is not known whether this drug is excreted in human milk. Caution should be exercised when administered to a nursing woman
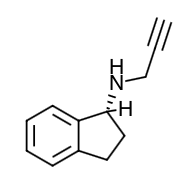
- Scientific Name: 1H-Inden-1-amine, 2, 3-dihydro-N-2-propynyl-, (1R)-, methanesulfonate
- Empirical Formula: (C12H13N)CH4SO3
- Molecular Weight: 267.34
- Rasagiline is a selective, irreversible MAO-B inhibitor indicated for the treatment of idiopathic Parkinson's disease. The results of a clinical trial designed to examine the effects of the drug on blood pressure when it is administered with increasing doses of tyramine indicates the functional selectivity can be incomplete when healthy subjects ingest large amounts of tyramine while receiving recommended doses of rasagiline. The selectivity for inhibiting MAO-B diminishes in a dose-related manner.
- MAO, a flavin-containing enzyme, is classified into two major molecular species, A and B, and is localized in mitochondrial membranes throughout the body in nerve terminals, brain, liver and intestinal mucosa. MAO regulates the metabolic degradation of catecholamines and serotonin in the CNS and peripheral tissues. MAO-B is the major form in the human brain. In ex vivo animal studies in brain, liver, and intestinal tissues, rasagiline was shown to be a potent, irreversible monoamine oxidase type B (MAO-B) selective inhibitor. Rasagiline at the recommended therapeutic dose was also shown to be a potent and irreversible inhibitor of MAO-B in platelets. The precise mechanisms of action of rasagiline are unknown. One mechanism is believed to be related to its MAO-B inhibitory activity, which causes an increase in extracellular levels of dopamine in the striatum. The elevated dopamine level and subsequent increased dopaminergic activity are likely to mediate rasagiline's beneficial effects seen in models of dopaminergic motor dysfunction.
- Tyramine Challenge Test: Results of a tyramine challenge study indicate that rasagiline at recommended doses is relatively selective for inhibiting MAO-B and can be used without dietary tyramine restriction. However, certain foods (e.g., aged cheeses, such as Stilton cheese) may contain very high amounts of tyramine (i.e., 150 mg or greater) and could potentially cause severe hypertension caused by tyramine interaction in patients taking rasagiline due to mild increased sensitivity to tyramine at recommended doses. Relative selectivity of rasagiline for inhibiting MAO-B diminished in a dose-related manner as the dose progressively increased above the highest recommended daily dose (1 mg).
- Platelet MAO Activity in Clinical Studies: Studies in healthy subjects and in Parkinson's disease patients have shown that rasagiline inhibits platelet MAO-B irreversibly. The inhibition lasts at least 1 week after last dose. Almost 25-35% MAO-B inhibition was achieved after a single rasagiline dose of 1 mg/day and more than 55% of MAO-B inhibition was achieved after a single rasagiline dose of 2 mg/day. Over 90% inhibition was achieved 3 days after rasagiline daily dosing at 2 mg/day and this inhibition level was maintained 3 days postdose. Multiple doses of rasagiline of 0.5, 1 and 2 mg per day resulted in complete MAO-B inhibition.
- Absorption: Rasagiline is rapidly absorbed, reaching peak plasma concentration (Cmax) in approximately 1 hour. The absolute bioavailability of rasagiline is about 36%. Food does not affect the Tmax of rasagiline, although Cmax and exposure (AUC) are decreased by approximately 60% and 20%, respectively, when the drug is taken with a high fat meal. Because AUC is not significantly affected, AZILECT can be administered with or without food.
- Distribution: The mean volume of distribution at steady-state is 87 L, indicating that the tissue binding of rasagiline is in excess of plasma protein binding. Plasma protein binding ranges from 88-94% with mean extent of binding of 61-63% to human albumin over the concentration range of 1-100 ng/mL.
- Metabolism: Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. The metabolism of rasagiline proceeds through two main pathways: N-dealkylation and/or hydroxylation to yield 1-aminoindan (AI), 3-hydroxy-N-propargyl-1 aminoindan (3-OH-PAI) and 3-hydroxy-1-aminoindan (3-OH-AI). In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP1A2 being the major isoenzyme involved in rasagiline metabolism. Glucuronide conjugation of rasagiline and its metabolites, with subsequent urinary excretion, is the major elimination pathway.
- Elimination: After oral administration of 14C-labeled rasagiline, elimination occurred primarily via urine and secondarily via feces (62% of total dose in urine and 7% of total dose in feces over 7 days), with a total calculated recovery of 84% of the dose over a period of 38 days. Less than 1% of rasagiline was excreted as unchanged drug in urine.
- Specific Populations
- Hepatic Impairment: Following repeat dose administration (7 days) of rasagiline (1 mg/day) in subjects with mild hepatic impairment (Child-Pugh score 5-6), AUC and Cmax were increased by 2 fold and 1.4 fold, respectively, compared to healthy subjects. In subjects with moderate hepatic impairment (Child-Pugh score 7-9), AUC and Cmax were increased by 7 fold and 2 fold, respectively, compared to healthy subjects.
- Renal Impairment: Following repeat dose administration (8 days) of rasagiline (1 mg/day) in subjects with moderate renal impairment, rasagiline exposure (AUC) was similar to rasagiline exposure in healthy subjects, while the major metabolite 1-AI exposure (AUC) was increased 1.5- fold in subjects with moderate renal impairment, compared to healthy subjects. Because 1-AI is not an MAO inhibitor, no dose adjustment is needed for patients with mild and moderate renal impairment. Data are not available for patients with severe renal impairment.
- Elderly: Since age has little influence on rasagiline pharmacokinetics, it can be administered at the recommended dose in the elderly (≥ 65 years).
- Pediatric: Rasagiline has not been investigated in patients below 18 years of age.
- Gender: The pharmacokinetic profile of rasagiline is similar in men and women.
- Drug-Drug Interactions
- Levodopa: A study in Parkinson's disease patients, in which the effect of levodopa/carbidopa (LD/CD) on rasagiline pharmacokinetics at steady state was investigated, showed that the pharmacokinetics of rasagiline were not affected by concomitant administration of LD/CD.
- Effect of Other Drugs on the Metabolism of rasagiline: In vitro metabolism studies showed that CYP1A2 was the major enzyme responsible for the metabolism of rasagiline. There is the potential for inhibitors of this enzyme to alter rasagiline clearance when coadministered.
- Ciprofloxacin: When ciprofloxacin, an inhibitor of CYP1A2, was administered to healthy volunteers (n=12) at 500 mg (BID) with rasagiline at 2 mg/day, the AUC of rasagiline increased by 83% and there was no change in the elimination half- life.
- Theophylline: Coadministration of rasagiline 1 mg/day and theophylline, a substrate of CYP1A2, up to 500 mg twice daily to healthy subjects (n=24) did not affect the pharmacokinetics of either drug.
- Antidepressants: Severe CNS toxicity (occasionally fatal) associated with hyperpyrexia as part of a serotonin syndrome, has been reported with combined treatment of an antidepressant (e.g., from one of many classes including tricyclic or tetracyclic antidepressants, SSRIs, SNRIs, triazolopyridine antidepressants) and nonselective MAOI or a selective MAO-B inhibitor.
- Effect of rasagiline on Other Drugs: No additional in vivo trials have investigated the effect of rasagiline on other drugs metabolized by the cytochrome P450 enzyme system. In vitro studies showed that rasagiline at a concentration of 1 mcg/ml (equivalent to a level that is 160 times the average Cmax ~ 5.9-8.5 ng/mL in Parkinson's disease patients after 1 mg rasagiline multiple dosing) did not inhibit cytochrome P450 isoenzymes, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A. These results indicate that rasagiline is unlikely to cause any clinically significant interference with substrates of these enzymes.
- Advise patients that treatment with recommended doses may be associated with elevations of blood pressure. Contact their healthcare provider if elevation of blood pressure occurs.
- Advise patients to avoid certain food (e.g., aged cheese) containing a very large amount of tyramine while take recommended doses of the drug because of the potential for large increases in blood pressure. Contact their healthcare provider if they eat foods very rich in tyramine and do not feel well soon after eating.
- Tell patients to inform their physician if they are taking, or planning to take, any prescription or over-the-counter drugs, especially antidepressants and over-the-counter cold medications, since there is a potential for interaction with rasagiline. They should contact their healthcare provider before taking analgesics.
- Advise patients about the potential for sedating effects associated with rasagiline, including somnolence and particularly to the possibility of falling asleep while engaged in activities of daily living. Patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with the drug to gauge whether or not it affects their mental and/or motor performance adversely.
- Advise patients to exercise caution when they are taking other sedating medications, alcohol, or other CNS depressants (e.g., benzodiazepines, antipsychotics, antidepressants) in combination with rasagiline.
- Inform patients that they should contact their healthcare provider if they take ciprofloxacin or a similar drug that could increase blood levels of rasagiline.
- Patients should be advised that they may develop orthostatic hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. These symptoms may occur more frequently during initial therapy or with an increase in dose at any time. Caution patients against standing up rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially, at the initiation of treatment.
- Advise patients taking rasagiline as adjunct to levodopa that there is a possibility of dyskinesia or increased dyskinesia.
- Inform patients that hallucinations or other manifestations of psychotic-like behavior can occur when taking rasagiline. Advise patients that, if they have a major psychotic disorder, that rasagiline should not ordinarily be used. Treatments for psychosis may decrease the effectiveness of rasagiline.
- Advise patients that they may experience intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges during treatment. Advise healthcare provider if any new urges develop or increase in frequency.
- Tell patients to contact their healthcare provider if they wish to discontinue treatment.
- Instruct patients to take the medicine as prescribed. If a doss is missed, the patient should not double the dose. The next dose should be taken at the usual time on the following day.
Dosing (Adult)
Hepatic Dosing
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|