Prasugrel (Effient): Drug Monograph
|
|---|
- Reduction of thrombotic cardiovascular events (including stent thrombosis) in patients with acute coronary syndrome who are to be managed with PCI as follows:
- Patients with unstable angina or non-ST-elevation myocardial infarction (NSTEMI)
- Patients with ST-elevation myocardial infarction (STEMI) when managed with either primary or delayed PCI
- General Dosing & Administration Notes:
- Patients should also take aspirin (75 mg to 325 mg daily).
- Take with or without food.
- Do not break the tablet.
- Acute Coronary Syndrome in PCI:
- Loading Dose: 60 mg by mouth as a single dose asap and no later than 1 hour after PCI
- Maintenance: 10 mg daily + aspirin for at least 12 months post stent placement
- If <60 kg: Consider lowering the maintenance dose to 5 mg daily
- Can cause significant, sometimes fatal, bleeding
- Do not use in patients with active pathological bleeding or a history of transient ischemic attack or stroke
- In patients ≥75 years of age, prasugrel is generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior MI) where its effect appears to be greater and its use may be considered.
- Do not start prasugrel in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue at least 7 days prior to any surgery.
- Additional risk factors for bleeding include: body weight <60 kg; propensity to bleed; concomitant use of medications that increase the risk of bleeding (e.g., warfarin, heparin, fibrinolytic therapy, chronic use of non-steroidal anti-inflammatory drugs [NSAIDs]).
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of prasugrel.
- If possible, manage bleeding without discontinuing prasugrel. Discontinuing, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular events.
- Active pathological bleeding
- Prior transient ischemic attach or stroke
- Hypersensitivity to Prasugrel or any component of the product
- CABG-related bleeding - risk increases in patients receiving prasugrel who undergo CABG
- Discontinuation of prasugrel - premature discontinuation increases risk of stent thrombosis, MI, and death
- Thrombotic thrombocytopenic purpura (TTP) - TTP has been reported with prasugrel
- Hypersensitivity - including angioedema in patients with a history of hypersensitivity reaction to other thienopyridines
- Bleeding - including life-threatening and fatal bleeding
- Thrombotic thrombocytopenic purpura
- Platelet inhibition by prasugrel is rapid and irreversible, lasting for the life of the platelet, and is unlikely to be increased in the event of an overdose.
- Symptoms in animals (dogs and rats) included emesis, increased serum alkaline phosphatase, hepatocellular atrophy, mydriasis, irregular respiration, decreased locomotor activity, ptosis, staggering gait, and lacrimation.
- Platelet transfusion may restore clotting ability.
- The prasugrel active metabolite is not likely to be removed by dialysis.
- Warfarin - increases the risk of bleeding
- Non-steroidal anti-inflammatory drugs - may increase the risk of bleeding
- Pregnancy: Pregnancy Category B
- Labor and Delivery: None
- Nursing Mothers: It is not known whether prasugrel is excreted in human milk. Should be used during nursing only if the potential benefit to the mother justifies the potential risk to the nursing infant.
- Renal Impairment: No dosage adjustment is necessary. There is limited experience in patients with end-stage renal disease, but such patients are generally at higher risk of bleeding.
- Hepatic Impairment: No dosage adjustment is necessary in patients with mild to moderate impairment (Child-Pugh Class A and B). The pharmacokinetics and pharmacodynamics of prasugrel in patients with severe hepatic disease have not been studied, but such patients are generally at higher risk of bleeding.
- Pediatric Patients: Safety and effectiveness in pediatric patients have not been established.
- Geriatric Patients: Due to the risk of bleeding and uncertainty in the effectiveness of the treatment, use in patients ≥75 years of age is generally not recommended, except in high-risk situations (diabetes and past history of myocardial infarction) where its effect appears to be greater and its use may be considered.
- It is not known whether prasugrel is excreted in human milk. Should be used during nursing only if the potential benefit to the mother justifies the potential risk to the nursing infant.
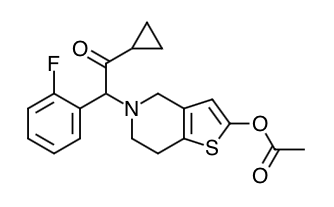
- Scientific Name: 5- [(1RS)-2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate hydrochloride
- Empirical Formula: C20H20FNO3S.HCI
- Molecular Weight: 409.90
- Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the PsY12 class of ADP receptors on platelets.
- Prasugrel produces inhibition of platelet aggregation to 20 μM or 5 μM ADP, as measured by light transmission aggregometry. Following a 60-mg loading dose, approximately 90% of patients had at least 50% inhibition of platelet aggregation by 1 hour. Maximum platelet inhibition was about 80%. Mean steady-state inhibition of platelet aggregation was about 70% following 3 to 5 days of dosing at 10-mg daily after a 60-mg loading dose.
- Platelet aggregation gradually returns to baseline values over 5-9 days after discontinuation of prasugrel, this time course being a reflection of new platelet production rather than pharmacokinetics of prasugrel. Discontinuing clopidogrel 75-mg and initiating a prasugrel 10-mg maintenance dose with or without a prasugrel 60-mg loading dose results in a decrease of 14 percentage points in maximum platelet aggregation (MPA) by Day 7. This decrease in MPA is not greater than that typically produced by a 10-mg maintenance dose of prasugrel alone. The relationship between inhibition of platelet aggregation and clinical activity has not been established.
- 5-mg in Low Body Weight Patients: In patients with stable coronary artery disease, mean platelet inhibition in subjects <60 kg taking 5-mg prasugrel was similar to that of subjects ≥60 kg taking 10-mg prasugrel. The relationship between inhibition of platelet aggregation and clinical activity has not been established.
- Prasugrel is a prodrug and is rapidly metabolized to a pharmacologically active metabolite and inactive metabolites. The active metabolite has an elimination half-life of about 7 hours (range 2-15 hours). Healthy subjects, patients with stable atherosclerosis, and patients undergoing PCI show similar pharmacokinetics.
- Absorption: Following oral administration, ≥79% of the dose is absorbed. The absorption and metabolism are rapid, with peak plasma concentrations (Cmax) of the active metabolite occurring approximately 30 minutes after dosing. The active metabolite's exposure (AUC) increases slightly more than proportionally over the dose range of 5 to 60-mg. Repeated daily doses of 10-mg do not lead to accumulation of the active metabolite. In a study of healthy subjects given a single 15-mg dose, the AUC of the active metabolite was unaffected by a high fat, high calorie meal, but Cmax was decreased by 49% and Tmax was increased from 0.5 to 1.5 hours. Effient can be administered without regard to food. The active metabolite is bound about 98% to human serum albumin.
- Distribution: (Active metabolit) Plasma protein binding (98%, albumin); Vd = 44-68L (active metabolite)
- Metabolism: Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel's active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins.
- Elimination: Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites. T1/2 = 7 hours (active metabolite)
- Specific Populations
- Pediatric: Pharmacokinetics and pharmacodynamics of prasugrel have not been evaluated in a pediatric population.
- Geriatric: In a study of 32 healthy subjects between the ages of 20 and 80 years, age had no significant effect on pharmacokinetics of prasugrel's active metabolite or its inhibition of platelet aggregation. In TRITON-TIMI 38, the mean exposure (AUC) of the active metabolite was 19% higher in patients ≥75 years of age than in patients <75 years of age. In a study in subjects with stable atherosclerosis, the mean exposure (AUC) to the active metabolite of prasugrel in subjects ≥75 years old taking a 5-mg maintenance dose was approximately half that seen in subjects 45 to 64 years old taking a 10-mg maintenance dose.
- Body Weight: The mean exposure (AUC) to the active metabolite is approximately 30 to 40% higher in subjects with a body weight of <60 kg than in those weighing ≥60 kg. In a study in subjects with stable atherosclerosis, the AUC of the active metabolite on average was 38% lower in subjects <60 kg taking 5-mg (N=34) than in subjects ≥60 kg taking 10- mg (N=38).
- Gender: Pharmacokinetics of prasugrel's active metabolite are similar in men and women.
- Ethnicity: Exposure in subjects of African and Hispanic descent is similar to that in Caucasians. In clinical pharmacology studies, after adjusting for body weight, the AUC of the active metabolite was approximately 19% higher in Chinese, Japanese, and Korean subjects than in Caucasian subjects.
- Smoking: Pharmacokinetics of prasugrel's active metabolite are similar in smokers and nonsmokers.
- Renal Impairment: Pharmacokinetics of prasugrel's active metabolite and its inhibition of platelet aggregation are similar in patients with moderate renal impairment (CrCL=30 to 50 mL/min) and healthy subjects. In patients with end- stage renal disease, exposure to the active metabolite (both Cmax and AUC (0-tlast) was about half that in healthy controls and patients with moderate renal impairment.
- Hepatic Impairment: Pharmacokinetics of prasugrel's active metabolite and inhibition of platelet aggregation were similar in patients with mild to moderate hepatic impairment compared to healthy subjects. The pharmacokinetics and pharmacodynamics of prasugrel's active metabolite in patients with severe hepatic disease have not been studied.
- Drug Interactions
- Inhibitors of CYP3A: Ketoconazole (400 mg daily), a selective and potent inhibitor of CYP3A4 and CYP3A5, did not affect prasugrel-mediated inhibition of platelet aggregation or the active metabolite's AUC and Tmax, but decreased the Cmax by 34% to 46%. Therefore, CYP3A inhibitors such as verapamil, diltiazem, indinavir, ciprofloxacin, clarithromycin, and grapefruit juice are not expected to have a significant effect on the pharmacokinetics of the active metabolite of prasugrel.
- Inducers of Cytochromes P450: Rifampicin (600 mg daily), a potent inducer of CYP3A and CYP2B6 and an inducer of CYP2C9, CYP2C19, and CYP2C8, did not significantly change the pharmacokinetics of prasugrel's active metabolite or its inhibition of platelet aggregation. Therefore, known CYP3A inducers such as rifampicin, carbamazepine, and other inducers of cytochromes P450 are not expected to have significant effect on the pharmacokinetics of the active metabolite of prasugrel.
- Drugs that Elevate Gastric pH: Daily coadministration of ranitidine (an H2 blocker) or lansoprazole (a proton pump inhibitor) decreased the Cmax of the prasugrel active metabolite by 14% and 29%, respectively, but did not change the active metabolite's AUC and Tmax. In TRITON-TIMI 38, Effient was administered without regard to coadministration of a proton pump inhibitor or H2 blocker.
- Statins: Atorvastatin (80 mg daily), a drug metabolized by CYP450 3A4, did not alter the pharmacokinetics of prasugrel's active metabolite or its inhibition of platelet aggregation.
- Heparin: A single intravenous dose of unfractionated heparin (100 U/kg) did not significantly alter coagulation or the prasugrel-mediated inhibition of platelet aggregation; however, bleeding time was increased compared with either drug alone.
- Aspirin: Aspirin 150 mg daily did not alter prasugrel-mediated inhibition of platelet aggregation; however, bleeding time was increased compared with either drug alone.
- Warfarin: A significant prolongation of the bleeding time was observed when prasugrel was coadministered with 15-mg of warfarin.
- Potential for Prasugrel to Affect Other Drugs: In vitro metabolism studies demonstrate that prasugrel's main circulating metabolites are not likely to cause clinically significant inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A, or induction of CYP1A2 or CYP3A.
- Drugs Metabolized by CYP2B6: Prasugrel is a weak inhibitor of CYP2B6. In healthy subjects, prasugrel decreased exposure to hydroxybupropion, a CYP2B6-mediated metabolite of bupropion, by 23%, an amount not considered clinically significant. Prasugrel is not anticipated to have significant effect on the pharmacokinetics of drugs that are primarily metabolized by CYP2B6, such as halothane, cyclophosphamide, propofol, and nevirapine.
- Effect on Digoxin: The potential role of prasugrel as a Pgp substrate was not evaluated. Prasugrel is not an inhibitor of Pgp, as digoxin clearance was not affected by prasugrel coadministration.
-
Summarize the effectiveness features and potential side effects of prasugrel.
-
Tell patients to take prasugrel exactly as prescribed. Remind patients not to discontinue the medication without first discussing it with the physician who prescribed it.
-
Inform patients that they will bruise and bleed more easily, will take longer than usual to stop bleeding, and should report any unanticipated, prolonged, or excessive bleeding, or blood in their stool or urine.
-
Inform patients that TTP is a rare but serious condition that has been reported with prasugrel and instruct patients to get prompt medical attention if they experience any of the following symptoms that cannot otherwise be explained: fever, weakness, extreme skin paleness, purple skin patches, yellowing of the skin or eyes, or neurological changes.
-
Inform patients that they may have hypersensitivity reactions including rash, angioedema, anaphylaxis, or other manifestations. Patients who have had hypersensitivity reactions to other thienopyridines may have hypersensitivity reactions to prasugrel.
-
Instruct patients to inform physicians and dentists that they are taking prasugrel before any invasive procedure is schedule. Instruct patients to tell the doctor performing the invasive procedure to talk to the prescribing health care professional before stopping prasugrel.
-
Ask patients to list all prescription medications, over-the-counter medications, or dietary supplements they are taking or plan to take so the physician knows about other treatments that may affect bleeding risk (e.g., warfarin and NSAIDs).
Indications
Dosing (Adult)
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





