Pitavastatin (Livalo): Drug Monograph
|
|---|
- General Dosing & Administration Notes:
- Take at any time of the day with or without food
- Primary Hyperlipidemia and Mixed Dyslipidemia:
- 2 mg by mouth
- Maximum dose: 4 mg daily
- After initiation or upon titration, analyze lipid levels after 4 weeks and adjust dose accordingly
- Moderate/severe (GFR 30-59 mL/min and GFR 15-29 mL/min not receiving hemodialysis):
- 1 mg by mouth daily
- Maximum: 2 mg daily
- ESRD on HD:
- 1 mg by mouth daily
- Maximum: 2 mg daily
- Known hypersensitivity to product components
- Active liver disease
- Women who are pregnant or may become pregnant
- Nursing mothers
- Co-administration with cyclosporine
- No know specific treatment in the event of overdose of pitavastatin.
- Treat patient symptomatically and use supportive measures as required.
- Erythromycin: Increases pitavastatin exposure. Limit pitavastatin to 1 mg once daily
- Rifampin: Increases pitavastatin exposure. Limit pitavastatin to 2 mg once daily.
- Concomitant lipid-lowering therapies: Increases the risk of adverse skeletal muscle effects. Use caution when prescribing pitavastatin.
- Pregnancy: Pregnancy category X
- Labor and Delivery: None
- Nursing Mothers: It is unknown whether pitavastatin is excreted in human milk. Women who require pitavastatin treatment should be advised not to nurse their infants or to discontinue pitavastatin.
- Renal Impairment: Limitation of a starting dose of pitavastatin 1 mg once daily and a maximum dose 2 mg once daily.
- Hepatic Impairment: Pitavastatin is contraindicated in patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels.
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: No significant differences in efficacy or safety between elderly and younger patients.
- It is unknown whether pitavastatin is excreted in human milk. Women who require pitavastatin treatment should be advised not to nurse their infants or to discontinue pitavastatin.
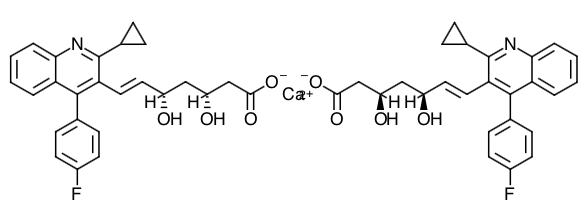
- Scientific Name: (+)monocalcium bis{(3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate}
- Empirical Formula: C50H46CaF2N2O8
- Molecular Weight: 880.98
- Pitavastatin competitively inhibits HMG-CoA reductase, which is a rate-determining enzyme involved with biosynthesis of cholesterol, in a manner of competition with the substrate so that it inhibits cholesterol synthesis in the liver. As a result, the expression of LDL-receptors followed by the uptake of LDL from blood to liver is accelerated and then the plasma TC decreases. Further, the sustained inhibition of cholesterol synthesis in the liver decreases levels of very low-density lipoproteins.
- In a randomized, double-blind, placebo-controlled, 4-way parallel, active-comparator study with moxifloxacin in 174 healthy participants, pitavastatin was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to16 mg (4 times the recommended maximum daily dose).
- Absorption: Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both Cmax and AUC0-inf increased in an approximately dose-proportional manner for single pitavastatin doses from 1 to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. Administration of pitavastatin with a high fat meal (50% fat content) decreases pitavastatin Cmax by 43% but does not significantly reduce pitavastatin AUC. The Cmax and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon.
- Distribution: Pitavastatin is more than 99% protein bound in human plasma, mainly to albumin and alpha 1-acid glycoprotein, and the mean volume of distribution is approximately 148 L. Association of pitavastatin and/or its metabolites with the blood cells is minimal.
- Metabolism: Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone which is formed via an ester-type pitavastatin glucuronide conjugate by uridine 5'-diphosphate (UDP) glucuronosyltransferase (UGT1A3 and UGT2B7).
- Elimination: A mean of 15% of radioactivity of orally administered single 32 mg 14C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days. The mean plasma elimination half-life is approximately 12 hours.
- Race: In pharmacokinetic studies pitavastatin Cmax and AUC were 21 and 5% lower, respectively in Black or African American healthy volunteers compared with those of Caucasian healthy volunteers. In pharmacokinetic comparison between Caucasian volunteers and Japanese volunteers, there were no significant differences in Cmax and AUC.
- Take pitavastatin at any time of the day with or without food
- Instruct patients to promptly notify their physician of any unexplained muscle pain, tenderness, or weakness particularly if accompanied by malaise or fever.
- Discuss all medication, both prescription and over the counter, with their physician.
- Women of childbearing age should use an effective method of birth control to prevent pregnancy while using pitavastatin.
- Inform women who are breastfeeding to not use pitavastatin.
- Instruct patients that liver enzyme tests be checked before the initiation of pitavastatin and if signs or symptoms of liver injury occur. Instruct patients to report promptly any symptoms that may include liver injury:
- Fatigue
- Anorexia
- Right upper abdominal discomfort
- Dark urine
- Jaundice
Dosing (Adult)
Renal Dosing
Contraindications
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





