Oxybate (Xyrem, GHB): Drug Monograph
|
|---|
- General Dosing & Administration Notes:
- Take 1st dose at least 2 hours after eating. Prepare both doses prior to bedtime. Prior to ingestion, dilute each dose with approximately 1.4 cup (approximately 60 mL) of water in the empty pharmacy vials provided. Take both doses while in bed and lie down immediately after dosing. Remain in bed following ingestion of the 1st and 2nd doses; an alarm may need to b e set to awaken for 2nd dose
- Narcolepsy:
- 4.5 g/night administered in 2 equally divided doses (given as 2.25 g at bedtime and 2.25 g taken 2.5 - 4 hours later)
- Increase by 1.5 g/night at weekly intervals (additional 0.75 g at bedtime and 0.75 g taken 2.5 - 4 hours later)
- Effective dose range: 6-9 g/night
- Maximum: 9 g/night
- Concomitant medications, divalproex sodium:
- Patients already stabilized on oxybate:
- Addition of divalproex sodium should be accompanied by an initial reduction in the nightly dose of oxybate by at least 20%
- Patients already taking divalproex sodium:
- Use lower starting dose of oxybate; monitor patient response and adjust dose accordingly
- Impairment:
-
2.25 g/night administered in 2 equally divided doses (approximately 1.13 g at bedtime and approximately 1.13 g taken 2.5-4 hours later)
- Obtundation and clinically significant respiratory depression may occur.
- Oxybate is the sodium salt of gamma hydroxybutyrate (GHB). Abuse of GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, and decreases in the level of consciousness, coma, and death.
- Prescribers and patients must enroll in the Xyrem REMS Program
- Succinic semialdehyde dehydrogenase deficiency
- In combination with sedative hypnotics or alcohol
- CNS depression - use caution when considering the concurrent use of oxybate with other CNS depressants
- Caution patients against hazardous activities requiring complete mental alertness or motor coordination within the first 6 hours of dosing or after first initiating treatment until certain that oxybate does not affect them adversely
- Respiratory depression and sleep-disordered breathing - drug may impair respiratory drive, especially in patients with compromised respiratory function
- Depression and suicidality - monitor patients for emergent or increased depression and suicidality
- Confusion/anxiety - monitor for impaired motor/cognitive function
- Parasomnias - evaluate episodes of sleepwalking
- High sodium content in oxybate - monitor patients with heart failure, hypertension, or impaired renal function
- Information regarding overdose is derived largely from reports in the medical literature that describe symptoms and signs in individuals who have ingested GHB illicitly
- Symptoms may include depressed consciousness that may fluctuate rapidly between a confusional, agitated combative state with ataxia and coma. Emesis (even when obtunded) diaphoresis, headache, and impaired psychomotor skills may be evident. Pupillary reactivity to light is maintained; blurred vision may occur. Respiration may be unaffected or compromised in rate and depth; Cheyne-Stokes respiration and apnea may occur. Bradycardia and hypothermia may accompany unconsciousness, as well as muscular hypotonia, but tendon reflexes remain intact.
- General symptomatic and supportive care should be instituted immediately and gastric decontamination may be considered if co-ingestants are suspected
- Appropriate posture (left lateral recumbent position) and protection of the airway by intubation may be warranted
- Monitor vital signs and consciousness
- The bradycardia reported with GHB overdose has been responsive to atropine IV administration; no reversal of the CNS effects can be expected from naloxone or flumazenil administration; the use of hemodialysis and other forms of extracorporeal drug removal have not been studied in overdose
- Alcohol, sedative hypnotics, and CNS depressants - should not b e used in combination with alcohol or hypnotics. Use of other CNS depressants may potentiate the CNS-depressant effects of oxybate.
- Divalproex sodium - results in a 25% mean increase in systemic exposure to oxybate (AUC ratio range of 0.8 to 1.7) and in a greater impairment on some tests of attention and working memory. Initial dose reduction of at least 20% of oxybate is recommended; monitor patient response closely and adjust dose accordingly.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: Has not been studied in labor or delivery
- Nursing Mothers: It is not known whether oxybate is excreted in human milk. Caution should be exercised when administered to a nursing woman
- Renal Impairment: None
- Hepatic Impairment: The starting dose should be reduced by 1/2 in patients with liver impairment
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: Dose selection should be cautious, usually starting at the low end of the dosing range
- It is not known whether oxybate is excreted in human milk. Caution should be exercised when administered to a nursing woman.
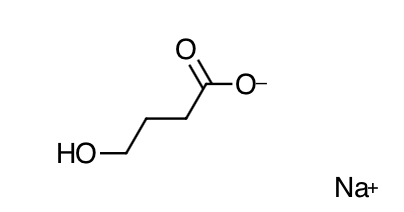
- Scientific Name: sodium 4-hydroxybutyrate
- Empirical Formula: C4H7NaO3
- Molecular Weight: 126.09
- Xyrem is a CNS depressant. The mechanism of action of Xyrem in the treatment of narcolepsy is unknown. Sodium oxybate is the sodium salt of gamma hydroxybutyrate, an endogenous compound and metabolite of the neurotransmitter GABA. It is hypothesized that the therapeutic effects of Xyrem on cataplexy and excessive daytime sleepiness are mediated through GABAB actions at noradrenergic and dopaminergic neurons, as well as at thalamocortical neurons
- Absorption: Following oral administration, sodium oxybate is absorbed rapidly across the clinical dose range, with an absolute bioavailability of about 88%. The average peak plasma concentrations (Cmax) following administration of each of the two 2.25 g doses given under fasting conditions 4 hours apart were similar. The average time to peak plasma concentration (Tmax) ranged from 0.5 to 1.25 hours. Following oral administration, the plasma levels of sodium oxybate increased more than dose-proportionally, with blood levels increasing 3.7-fold as total daily dose is doubled from 4.5 g to 9 g. Single doses greater than 4.5 g have not been studied. Administration of oxybate immediately after a high-fat meal resulted in delayed absorption (average Tmax increased from 0.75 hour to 2 hours) and a reduction in Cmax by a mean of 59% and of systemic exposure (AUC) by 37%.
- Distribution: Sodium oxybate is a hydrophilic compound with an apparent volume of distribution averaging 190 mL/kg to 384 mL/kg. At sodium oxybate concentrations ranging from 3 mcg/mL to 300 mcg/mL, less than 1% is bound to plasma proteins.
- Metabolism: Animal studies indicate that metabolism is the major elimination pathway for sodium oxybate, producing carbon dioxide and water via the tricarboxylic acid (Krebs) cycle and secondarily by beta-oxidation. The primary pathway involves a cytosolic NADP+ linked enzyme, GHB dehydrogenase, that catalyzes the conversion of sodium oxybate to succinic semialdehyde, which is then biotransformed to succinic acid by the enzyme succinic semialdehyde dehydrogenase. Succinic acid enters the Krebs cycle where it is metabolized to carbon dioxide and water. A second mitochondrial oxidoreductase enzyme, a transhydrogenase, also catalyzes the conversion to succinic semialdehyde in the presence of -ketoglutarate. An alternate pathway of biotransformation involves -oxidation via 3,4-dihydroxybutyrate to carbon dioxide and water. No active metabolites have been identified.
- Elimination: The clearance of sodium oxybate is almost entirely by biotransformation to carbon dioxide, which is then eliminated by expiration. On average, less than 5% of unchanged drug appears in human urine within 6 to 8 hours after dosing. Fecal excretion is negligible. Sodium oxybate has an elimination half-life of 0.5-1 hour.
- Specific Populations:
- Geriatric: There is limited experience with oxybate in the elderly. Results from a pharmacokinetic study (n=20) in another studied population indicate that the pharmacokinetic characteristics of sodium oxybate are consistent among younger (age 48-64 years) and older (age 65-75 year) adults.
- Pediatric: The pharmacokinetics in patients younger than 18 years of age have not been studied.
- Gender: In a study of 18 female and 18 male healthy adult volunteers, no gender differences were detected in the pharmacokinetics of the oral solution following a single oral dose of 4.5 g.
- Race: There are insufficient data to evaluate any pharmacokinetic differences among races.
- Renal Impairment: No pharmacokinetic study in patients with renal impairment have been conducted.
- Hepatic Impairment: The pharmacokinetics of oxybate in 16 cirrhotic patients, half without ascites (Child's Class A) and half with ascites (Child's Class C), were compared to the kinetics in 8 subjects with normal hepatic function after a single oral dose of 25 mg/kg. AUC values were double in the cirrhotic patients, with apparent oral clearance reduced from 9.1 mL/min/kg in healthy adults to 4.5 and 4.1 mL/min/kg in Class A and Class C patients, respectively. Elimination half-life was significantly longer in Class C and Class A patients than in control patients (mean t 1/2 of 59 and 32 minutes, respectively, versus 22 minutes). The starting dose of oxybate should be reduced by one-half in patients with liver impairment.
- Drug Interaction Studies: Studies in vitro with pooled human liver microsomes indicate that sodium oxybate does not significantly inhibit the activities of the human isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A up to the concentration of 3 mM (378 mcg/mL), a level considerably higher than levels achieved with therapeutic doses. Drug interaction studies in healthy adults (age 18 to 50 years) were conducted with Xyrem and divalproex sodium, diclofenac, and ibuprofen:
- Divalproex sodium: Co-administration of oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with divalproex sodium (valproic acid, 1250 mg per day) increased mean systemic exposure to sodium oxybate as shown by AUC by approximately 25% , while Cmax was comparable. Co-administration did not appear to affect the pharmacokinetics of valproic acid. A greater impairment on some tests of attention and working memory was observed with co-administration of both drugs than with either drug alone.
- Diclofenac: Co-administration of oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with diclofenac (50 mg/dose twice per day) showed no significant differences in systemic exposure to sodium oxybate. Co-administration did not appear to affect the pharmacokinetics of diclofenac.
- Ibuprofen: Co-administration of oxybate (6 g per day as two equal doses of 3 grams dosed four hours apart) with ibuprofen (800 mg/dose four times per day also dosed four hours apart) resulted in comparable systemic exposure to sodium oxybate as shown by plasma Cmax and AUC values. Co-administration did not affect the pharmacokinetics of ibuprofen.
- Drug interaction studies in healthy adults demonstrated no pharmacokinetic interactions between sodium oxybate and protriptyline hydrochloride, zolpidem tartrate, and modafinil. Also, there were no pharmacokinetic interactions with the alcohol dehydrogenase inhibitor fomepizole. However, pharmacodynamic interactions with these drugs cannot be ruled out. Alteration of gastric pH with omeprazole produced no significant change in the oxybate kinetics. In addition, drug interaction studies in healthy adults demonstrated no pharmacokinetic or clinically significant pharmacodynamic interactions between sodium oxybate and the SNRI duloxetine HCl.
- Inform patients that oxybate is available only through a restricted distribution program called the Xyrem REMS program. Patients musty read and understand the materials in the program prior to initiating treatment. Inform the patient that they should be seen by the prescriber frequently to review dose titration, symptom response, and adverse reactions; a follow-up of every three months is recommended.
- Discuss safe and proper use of oxybate and dosing information with patients prior to the initiation of treatment. Instruct patients to store oxybate bottles and doses in a secure place, out of the reach of children and pets.
- Advise patients not to drink alcohol or take other sedative hypnotics.
- Inform patients that after taking oxybate they are likely to fall asleep quickly (often within 4 and usually within 15 minutes), but the time it takes to fall asleep can vary from night to night. The sudden onset of sleep, including in a standing position or while rising from bed, has led to falls and injuries. Instruct patients to remain in bed following ingestion of the first and second doses. Instruct patients not to take their second dose until 2.5 to 4 hours after the first dose.
- Inform patients to take the first dose at least 2 hours after eating.
- Inform patients that oxybate can be associated with respiratory depression.
- Inform patients that until they are reasonably certain that oxybate does not affect them adversely (e.g., impair judgment, thinking, or motor skills) they should not operate hazardous machinery, including automobiles or airplanes.
- Instruct patients or families to contact a healthcare provider immediately if the patient develops depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or suicidal ideation.
- Instruct patients and their families that oxybate has been associated with sleepwalking and to contact their healthcare provider if this occurs.
- Instruct patients who are sensitive to salt intake (e.g., those with heart failure, hypertension, or renal impairment) that oxybate contains a significant amount of sodium and they should limit their sodium intake.
Dosing (Adult)
Hepatic Dosing
Black Box Warnings
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

