Labetalol (Normodyne, Trandate): Drug Monograph
|
|---|
- Management of hypertension. May be used alone or in combination with other antihypertensive agents, especially thiazide and loop diuretics.
- Should side effects occur, same total daily dose administered three times a day may improve tolerability and facilitate further titration.
- Titration increments should not exceed 200 mg twice daily.
- Hypertension:
- 100 mg twice daily whether used alone or added to a diuretic regimen
- After 2 or 3 days may be titrated in increments of 100 mg twice daily every 2-3 days
- Maintenance: 200-400 mg twice daily
- Severe Hypertension:
- 1200-2400 mg daily given twice a day with or without diuretics.
- Acute Hypertension or Hypertensive Crisis:
- Acute:
- 10-20 mg IV push for 2 minutes initially
- Increase 40-80 mg every 10 minutes
- Total maximum dose 300 mg
- Maintenance:
- .5-2 mg IV infusion per minute
- Total maximum dose 300 mg
- Conversion from IV to PO (Estimated Recommendations):
- Initial oral dose 200 mg
- Additional dose of 200-400 mg in 6-12 hours
- Maintenance: 400-2,400 mg daily given twice a day
- Bronchial asthma
- Overt cardiac failure
- Greater-than-first-degree heart block
- Cardiogenic shock
- Severe bradycardia
- Other conditions associated with severe and prolonged hypotension
- History of hypersensitivity to any component of the product
- History of obstructive airway disease, including asthma
-
Hepatic injury
-
Cardiac failure
-
Exacerbation of ischemic heart disease following abrupt withdrawal
-
Nonallergic bronchospasm (e.g., chronic bronchitis and emphysema) - do not use with patients with bronchospastic disease
-
Pheochromocytoma - use caution when administering
-
Diabetes mellitus and hypoglycemia
-
Major surgery - do not routinely withdraw chronic beta blocker therapy prior to surgery
-
Impaired hepatic function - use with caution in patients with impaired hepatic function
-
Jaundice or hepatic dysfunction
- Fatigue
- Asthenia
- Headache
- Nausea
- Dyspepsia
- Vomiting
- Diarrhea
- Taste distortion
- Dizziness
- Nasal stuffiness
- Ejaculation failure, impotence
- Edema
- Postural hypotension
- Dyspnea
- Rash
- Vision abnormality
- Vertigo
- Excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia are signs of overdosage.
- Patients should be placed supine and their legs raised if necessary to improve the blood supply to the brain.
- Gastric lavage or pharmacologically induced emesis (using syrup of ipecac) may be used for removal of the drug shortly after oral ingestion.
- If necessary, administer atropine or epinephrine for excessive bradycardia.
- If necessary, administer a digitalis glycoside and a diuretic (dopamine or dobutamine may be useful also) for cardiac failure.
- If necessary, administer epinephrine and/or an aerosolized beta2-agonist for bronchospasm.
- Tricyclic antidepressants - may experience tremor
- Beta-receptor agonist drugs in patients with bronchospasm - greater doses than normal of the beta-agonist drug may be required
- Cimetidine
- Halothane anesthesia - notify anesthesiologist when a patient is receiving labetalol
- Nitroglycerin - additional antihypertensive effects may occur
- Calcium antagonists of the verapamil type
- Digitalis glycosides - increase risk of bradycardia
-
Pregnancy: Pregnancy Category C
-
Labor and Delivery: None
-
Nursing Mothers: Small amounts are excreted in human milk. Use caution when administered to a nursing woman.
-
Renal Impairment: None
-
Hepatic Impairment: None
-
Pediatric Patients: Safety and effectiveness have not been established.
-
Geriatric Patients: Caution elderly patients about the possibility of orthostatic symptoms during treatment. Elimination of labetalol is reduced in elderly patients. May require a lower maintenance dosage (100-200 mg twice daily) than nonelderly patients.
- Small amounts are excreted in human milk. Use caution when administered to a nursing woman.
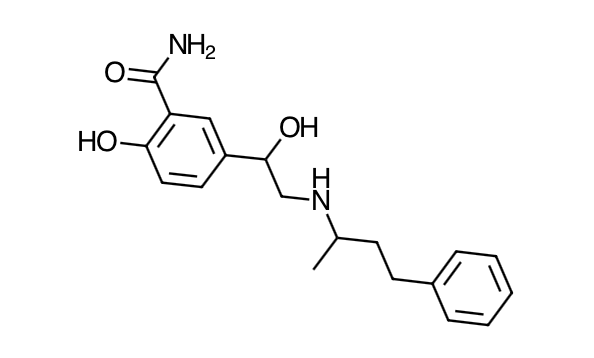
- Scientific Name: 2-hydroxy-5-[1-hydroxy-2-[(1 methyl-3-phenylpropyl)amino]ethyl]benzamide monohydrochloride
- Empirical Formula: C19H24N2O3,HCl
- Molecular Weight: 364.9
- Labetalol HCl combines both selective, competitive, alpha1 -adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous (IV) administration, respectively. Beta2 -agonist activity has been demonstrated in animals with minimal beta1 -agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane stabilizing effect has been demonstrated.
- The capacity of labetalol HCl to block alpha receptors in man has been demonstrated by attenuation of the pressor effect of phenylephrine and by a significant reduction of the pressor response caused by immersing the hand in ice-cold water ("cold-pressor test"). Labetalol HCl's beta1 -receptor blockade in man was demonstrated by a small decrease in the resting heart rate, attenuation of tachycardia produced by isoproterenol or exercise, and by attenuation of the reflex tachycardia to the hypotension produced by amyl nitrite. Beta2 -receptor blockade was demonstrated by inhibition of the isoproterenol-induced fall in diastolic blood pressure. Both the alpha- and beta-blocking actions of orally administered labetalol HCl contribute to a decrease in blood pressure in hypertensive patients. Labetalol HCl consistently, in dose-related fashion, blunted increases in exercise-induced blood pressure and heart rate, and in their double product. The pulmonary circulation during exercise was not affected by labetalol HCl dosing.
- Single oral doses of labetalol HCl administered to patients with coronary artery disease had no significant effect on sinus rate, intraventricular conduction, or QRS duration. The atrioventricular (A-V) conduction time was modestly prolonged in two of seven patients. In another study, IV labetalol HCl slightly prolonged A-V nodal conduction time and atrial effective refractory period with only small changes in heart rate. The effects on A-V nodal refractoriness were inconsistent.
- Labetalol HCl produces dose-related falls in blood pressure without reflex tachycardia and without significant reduction in heart rate, presumably through a mixture of its alpha- and beta-blocking effects. Hemodynamic effects are variable, with small, nonsignificant changes in cardiac output seen in some studies but not others, and small decreases in total peripheral resistance. Elevated plasma renins are reduced.
- Doses of labetalol HCl that controlled hypertension did not affect renal function in mildly to severely hypertensive patients with normal renal function.
- Due to the alpha1-receptor blocking activity of labetalol HCl, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension (2%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when the recommended starting dose and titration increments are closely followed. Symptomatic postural hypotension is most likely to occur 2 to 4 hours after a dose, especially following the use of large initial doses or upon large changes in dose.
- The peak effects of single oral doses of labetalol HCl occur within 2 to 4 hours. The duration of effect depends upon dose, lasting at least 8 hours following single oral doses of 100 mg and more than 12 hours following single oral doses of 300 mg. The maximum, steady-state blood pressure response upon oral, twice-a-day dosing occurs within 24 to 72 hours.
- The antihypertensive effect of labetalol has a linear correlation with the logarithm of labetalol plasma concentration, and there is also a linear correlation between the reduction in exercise-induced tachycardia occurring at 2 hours after oral administration of labetalol HCl and the logarithm of the plasma concentration.
- About 70% of the maximum beta-blocking effect is present for 5 hours after the administration of a single oral dose of 400 mg with suggestion that about 40% remains at 8 hours.
- The antianginal efficacy of labetalol HCl has not been studied. In 37 patients with hypertension and coronary artery disease, labetalol HCl did not increase the incidence or severity of angina attacks.
- Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache, and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta receptors.
- Although beta-adrenergic receptor blockade is useful in the treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen A-V block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta2 -adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm, and it may also interfere with exogenous bronchodilators in such patients.
- Absorption: Labetalol HCl is completely absorbed from the gastrointestinal tract with peak plasma levels occurring 1 to 2 hours after oral administration. The relative bioavailability of labetalol HCl tablets compared to an oral solution is 100%. The absolute bioavailability (fraction of drug reaching systemic circulation) of labetalol when compared to an IV infusion is 25%; this is due to extensive "first-pass" metabolism. Despite "first-pass" metabolism, there is a linear relationship between oral doses of 100 to 3,000 mg and peak plasma levels. The absolute bioavailability of labetalol is increased when administered with food. The plasma half-life of labetalol following oral administration is about 6 to 8 hours. Steady-state plasma levels of labetalol during repetitive dosing are reached by about the third day of dosing. In patients with decreased hepatic or renal function, the elimination half-life of labetalol is not altered; however, the relative bioavailability in hepatically impaired patients is increased due to decreased "first-pass" metabolism.
- Distribution: Plasma protein binding (50%); found in breast milk; crosses placenta
- Metabolism: The metabolism of labetalol is mainly through conjugation to glucuronide metabolites. These metabolites are present in plasma and are excreted in the urine and, via the bile, into the feces.
- Elimination: Approximately 55% to 60% of a dose appears in the urine as conjugates or unchanged labetalol within the first 24 hours of dosing. Labetalol has been shown to cross the placental barrier in humans. Only negligible amounts of the drug crossed the blood-brain barrier in animal studies. Labetalol is approximately 50% protein bound. Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol HCl from the general circulation (<1%). Some pharmacokinetic studies indicate that the elimination of labetalol is reduced in elderly patients.
- Advise patients to take exactly as prescribed. Treatment should not be interrupted or discontinued without a physician's advice.
- Advise patients to consult a physician at any signs or symptoms of impending cardiac failure or hepatic dysfunction.
- Advise patients that transient scalp tingling may occur, especially when treatment is initiated.
- Advise patients with concomitant illnesses such as impaired renal function, that appropriate tests should be done to monitor these conditions over regular intervals of treatment with labetalol.
Indications
Dosing (Adult)
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

