Ivabradine (Corlanor): Drug Monograph
|
|---|
- To reduce the risk of hospitalization for worsening heart failure (HF) in patients with stable, symptomatic chronic HF with left ventricular ejection fraction ≤35%, who are in sinus rhythm with resting HR ≥70 bpm and either are on maximally tolerated doses of β-blockers or have a contraindication to β-blocker use.
- Take with meals
- Heart Failure:
-
5 mg twice daily
-
After 2 weeks, assess patient and adjust dose to achieve a resting HR of 50-60 bpm
-
Maximum: 7.5 mg twice daily
-
HR <50 bpm or signs/symptoms of bradycardia: Decrease dose by 2.5 mg (given twice daily); if current dose is 2.5 mg twice a day, discontinue therapy
-
HR 50-60 bpm : Maintain dose
-
HR >60 bpm: Increase dose by 2.5 mg (given twice daily) up to a maximum dose of 7.5 mg twice daily. Thereafter, adjust dose as needed based on resting HR and tolerability
- History of conduction defects or bradycardia leading to hemodynamic compromise:
- 2.5 mg twice daily
- Increase dose based on HR
- Acute decompensated heart failure
- Blood pressure <90/50 mmHg
- Sick sinus syndrome, sinoatrial block or 3rd degree AV block, unless a functioning demand pacemaker is present
- Resting heart rate <60 bpm prior to treatment
- Severe hepatic impairment
- Pacemaker dependence (heart rate maintained exclusively by the pacemaker)
- In combination with strong cytochrome CYP3A4 inhibitors
- Fetal toxicity - females should use effective contraception
- Monitor patients for atrial fibrillation
- Monitor heart rate decreases and bradycardia symptoms during treatment
- Not recommended in patients with 2nd degree AV block
- May lead to severe and prolonged bradycardia.
- In the event of bradycardia with poor hemodynamic tolerance, temporary cardiac pacing may be required.
- Supportive treatment, including IV fluids, atropine, and IV beta-stimulating agents such as isoproterenol, may be considered.
- CYP3A4 inhibitors - increase ivabradine plasma concentrations; avoid use of moderate CYP3A4 inhibitors (i.e., diltiazem, verapamil, and grapefruit juice)
- CYP3A4 inducers - decrease ivabradine plasma concentrations; avoid use of moderate inducers (i.e., St John's Wort, rifampicin, barbiturates, and phenytoin)
- Negative chronotropes - increased risk of bradycardia, monitor heart rate
- Pacemakers - not recommended for use with demand pacemakers set to rates ≥60 beats per minute
- Pregnancy: Pregnancy Category not established; advise a pregnant woman of the potential risk to the fetus. Pregnant patients who are started on treatment, especially during the 1st trimester, should be followed closely for destabilization of heir CHF that could result from HR slowing; monitor pregnant women with chronic HF in 3rd trimester of pregnancy for preterm birth
- Labor and Delivery: None
- Nursing Mothers: No information regarding the presence of ivabradine in human milk; breastfeeding is not recommended
- Renal Impairment: No dosage adjustment required for patients with CrCl 15-60 mL/min; no data available for CrCl <15 mL/min
- Hepatic Impairment: No dose adjustment required with mild or moderate impairment' contraindicated in patients with severe impairment
- Pediatric Patients: Safety and effectiveness have not been established
- Geriatric Patients: No pharmacokinetic differences have been observed in elderly or very elderly patients compared to the overall population
- No information regarding the presence of ivabradine in human milk; breastfeeding is not recommended
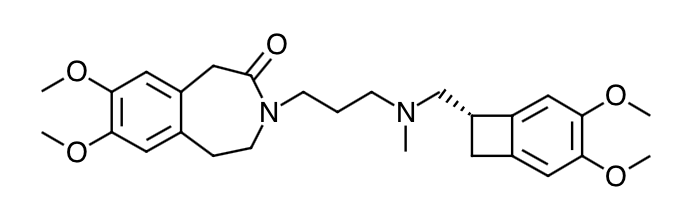
- Scientific Name: 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7- yl)methyl] methyl amino} propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride
- Empirical Formula: C27H36N2O5,HCL
- Molecular Weight: 505.1 (free base + HCL)
- Ivabradine blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel responsible for the cardiac pacemaker If current, which regulates heart rate. In clinical electrophysiology studies, the cardiac effects were most pronounced in the sinoatrial (SA) node, but prolongation of the AH interval has occurred on the surface ECG, as has PR interval prolongation. There was no effect on ventricular repolarization and no effects on myocardial contractility. It can also inhibit the retinal current Ih. Ih is involved in curtailing retinal responses to bright light stimuli. Under triggering circumstances (e.g., rapid changes in luminosity), partial inhibition if Ih by ivabradine may underlie the luminous phenomena experienced by patients. Luminous phenomena (phosphenes) are described as a transient enhanced brightness in a limited area of the visual field.
- Ivabradine causes a dose-dependent reduction in heart rate. The size of the effect is dependent on the baseline heart rate (i.e., greater heart rate reduction occurs in subjects with higher baseline heart rate). At recommended doses, heart rate reduction is approximately 10 bpm at rest and during exercise. Analysis of heart rate reduction vs. dose indicates a plateau effect at doses > 20 mg twice daily. In a study of subjects with preexisting conduction system disease (first- or second-degree AV block or left or right bundle branch block) requiring electrophysiologic study, IV ivabradine (0.20 mg/kg) administration slowed the overall heart rate by approximately 15 bpm, increased the PR interval (29 msec), and increased the AH interval (27 msec). Ivabradine does not have negative inotropic effects. It increases the uncorrected QT interval with heart rate slowing but does not cause rate-corrected prolongation of QT.
- Absorption: Following oral administration, peak plasma ivabradine concentrations are reached in approximately 1 hour under fasting conditions. The absolute oral bioavailability of ivabradine is approximately 40% because of first-pass elimination in the gut and liver. Food delays absorption by approximately 1 hour and increases plasma exposure by 20% to 40%. Ivabradine should be taken with meals.
- Distribution: Ivabradine is approximately 70% plasma protein bound, and the volume of distribution at steady state is approximately 100 L.
- Metabolism: The pharmacokinetics of ivabradine are linear over an oral dose range of 0.5 mg to 24 mg. Ivabradine is extensively metabolized in the liver and intestines by CYP3A4-mediated oxidation. The major metabolite is the N-desmethylated derivative (S 18982), which is equipotent to ivabradine and circulates at concentrations approximately 40% that of ivabradine. The N-desmethylated derivative is also metabolized by CYP3A4. Ivabradine plasma levels decline with a distribution half-life of 2 hours and an effective half-life of approximately 6 hours.
- Elimination: The total clearance of ivabradine is 24 L/h, and renal clearance is approximately 4.2 L/h, with ~ 4% of an oral dose excreted unchanged in urine. The excretion of metabolites occurs to a similar extent via feces and urine.
- Specific Populations
- Age: No pharmacokinetic differences (AUC or Cmax) have been observed between elderly (≥65 years) or very elderly (≥75 years) patients and the overall patient population.
- Hepatic Impairment: In patients with mild (Child-Pugh A) and moderate (Child-Pugh B) hepatic impairment, the pharmacokinetics of ivabradine were similar to that in patients with normal hepatic function. No data are available in patients with severe hepatic impairment (Child-Pugh C).
- Renal Impairment: Renal impairment (creatinine clearance from 15 to 60 mL/min) has minimal effect on the pharmacokinetics. No data are available for patients with creatinine clearance below 15 mL/min.
- Pediatrics: The pharmacokinetics have not been investigated in patients < 18 years of age.
- Advise pregnant women of the potential risks to a fetus; advise females of reproductive potential to use effective contraception and to notify their healthcare provider with a known or suspected pregnancy.
- Advise patients to report significant decreases in heart rate or symtpoms such as dizziness, fatigue, or hypotension.
- Advise patients to report symptoms of atrial fibrillation, such as heart palpitations or racing, chest pressure, or worsened shortness of breath.
- Counsel patients about the possible occurrence of luminous phenomenon (phosphenes). Advise patients to use caution if they are driving or using machines in situation where sudden changes in light intensity may occur, especially when driving at night. Advise patients that phosphenes may subside spontaneously during continued treatment with ivabradine.
- Advise patients to avoid ingestion of grapefruit juice and St. John's Wort.
- Counsel patients to take ivabradine twice daily with meals.
Indications
Dosing (Adult)
General Dosing & Administration Notes:
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|