Ezetimibe (Zetia): Drug Monograph
|
|---|
- An adjunct to diet to:
- Reduce elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with primary hyperlipidemia, alone or in combination with an HMG-CoA reductase inhibitor (statin)
- Reduce elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with mixed hyperlipidemia in combination with fenofibrate
- Reduce elevated total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH), in combination with atorvastatin or simvastatin
- Reduce elevated sitosterol and campesterol in patients with homozygous sitosterolemia (phytosterolemia)
- Limitations of Use
- The effect of ezetimibe on cardiovascular morbidity and mortality has not been determined.
- Ezetimibe has not been studied in Fredrickson Type I, III, IV, and V dyslipidemias.
- General Notes:
- May be taken with or without food.
- Dosing of exetimibe should occur either ≥2 hours before or ≥4 hours after administration of a bile acid sequestrant.
- 10 mg once daily
- None
- Moderate to severe (GFR <60mL/min): Use caution and monitor closely with simvastatin doses >20 mg
- Statin contraindications apply when ezetimibe is used with a statin:
- Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels
- Women who are pregnant or may become pregnant
- Nursing mothers
- Known hypersensitivity to product components
- Moderate or severe hepatic impairment - not recommended
- Liver enzyme abnormalities and monitoring - persistent elevations in hepatic transaminase can occur when ezetimibe is added to a statin
- Skeletal muscle effects - myopathy and rhabdomyolysis. Risk for skeletal muscle toxicity increases with higher doses of statin, advanced age (>65), hypothyroidism, renal impairment, and depending on the statin used, concomitant use of other drugs.
- Nasopharyngitis
- Myalgia
- Upper respiratory tract infection
- Arthralgia
- Diarrhea
- Sinusitis
- Pain in extremity
- No reported clinical or laboratory adverse events.
- Symptomatic and supportive measures should be employed.
- Cyclosporine - increases exposure of ezetimibe and cyclosporine
- Fenofibrate - increases exposure of ezetimibe
- Fibrates - co-administration with fibrates other than fenofibrate is not recommended
- Cholestyramine - decreases exposure of ezetimibe
- Coumarin anticoagulants (warfarin) - the International Normalized Ratio (INR) should be appropriately monitored.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether ezetimibe is excreted into human breast milk. If used, the potential benefit should justify the potential risk to the infant.
- Renal Impairment: When used as mono-therapy, no dosage adjustment is necessary.
- Hepatic Impairment: Moderate to severe impairment: ezetimibe is not recommended.
- Pediatric Patients: Based on total ezetimibe (ezetimibe + ezetimibe-glucuronide), there are no pharmacokinetic differences between adolescents and adults. Pharmacokinetic data in the pediatric population <10 years of age are not available.
- Geriatric Patients: No overall differences in safety and effectiveness between elderly patients (65 years and older) and younger patients. Greater sensitivity of some older individuals cannot be ruled out.
- It is not known whether ezetimibe is excreted into human breast milk. If used, the potential benefit should justify the potential risk to the infant.
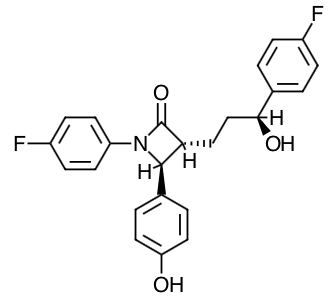
- Scientific Name: 1-(4-fluorophenyl)-3(R)-[3-(4-flurophenyl)-3(S)-hydroxypropyl}-4(S)-(4-hydroxyphenyl)-2-azetidinone.
- Empirical Formula: C24H21F2NO3
- Molecular Weight: 409.4
- Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine because of its inhibition of the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is involved in the intestinal uptake of cholesterol and phytosterols.
- Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion. Instead, ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver.
- In a 2-week clinical study in 18 hypercholesterolemic patients, ezetimibe inhibited intestinal cholesterol absorption by 54%, compared with placebo. Ezetimibe had no clinically meaningful effect on the plasma concentrations of the fat-soluble vitamins A, D, and E (in a study of 113 patients), and did not impair adrenocortical steroid hormone production (in a study of 118 patients).
- The cholesterol content of the liver is derived predominantly from three sources. The liver can synthesize cholesterol, take up cholesterol from the blood from circulating lipoproteins, or take up cholesterol absorbed by the small intestine. Intestinal cholesterol is derived primarily from cholesterol secreted in the bile and from dietary cholesterol.
- Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion. Instead, ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of statins and of fenofibrate.
- In a 2-week clinical study in 18 hypercholesterolemic patients, ezetimibe inhibited intestinal cholesterol absorption by 54%, compared with placebo. Ezetimibe had no clinically meaningful effect on the plasma concentrations of the fat-soluble vitamins A, D, and E (in a study of 113 patients), and did not impair adrenocortical steroid hormone production (in a study of 118 patients)
- Clinical studies have demonstrated that elevated levels of total-C, LDL-C and Apo B, the major protein constituent of LDL, promote human atherosclerosis. In addition, decreased levels of HDL-C are associated with the development of atherosclerosis.
- Ezetimibe reduces total-C, LDL-C, Apo B, non-HDL-C, and TG, and increases HDL-C in patients with hyperlipidemia.
- Administration of ezetimibe with a statin is effective in improving serum total-C, LDL-C, Apo B, non-HDL-C, TG, and HDL-C beyond either treatment alone.
- Administration of ezetimibe with fenofibrate is effective in improving serum total-C, LDL-C, Apo B, and non-HDL-C in patients with mixed hyperlipidemia as compared to either treatment alone.
- The effects of ezetimibe given either alone or in addition to a statin or fenofibrate on cardiovascular morbidity and mortality have not been established.
- Absorption:
- After oral administration, ezetimibe is absorbed and extensively conjugated to a pharmacologically active phenolic glucuronide (ezetimibe-glucuronide).
- The absolute bioavailability of ezetimibe cannot be determined, as the compound is virtually insoluble in aqueous media suitable for injection.
- Effect of Food on Oral Absorption:
- Concomitant food administration (high-fat or non-fat meals) had no effect on the extent of absorption of ezetimibe when administered as 10-mg tablets.
- Ezetimibe can be administered with or without food.
- Protein Binding:
- Ezetimibe and ezetimibe-glucuronide are highly bound (>90%) to human plasma protein.
- Metabolism:
- Ezetimibe is primarily metabolized in the small intestine and liver via glucuronide conjugation (a phase II reaction) with subsequent biliary and renal excretion. Minimal oxidative metabolism (a phase I reaction) has been observed in all species evaluated.
- Elimination:
- Ezetimibe was the major component in feces and accounted for 69% of the administered dose, while ezetimibe-glucuronide was the major component in urine and accounted for 9% of the administered dose.
- Half-Life: Approximately 22 hours
- Specific Populations:
- Geriatric Patients: In a multiple-dose study with ezetimibe given 10 mg daily for 10 days, plasma concentration for total ezetimibe were about 2-fold higher in older (≥65 years) healthy subjects compared to younger subjects.
- Gender: In a multiple-dose study with ezetimibe given 10 mg once daily for 10 days, plasma concentrations for total ezetimibe were slightly higher (>20%) in women than in men.
- Race: Based on a meta-analysis of multiple-dose pharmacokinetic studies, there were no pharmacokinetic differences between Black and Caucasian subjects. Studies in Asian subjects indicated that the pharmacokinetics of ezetimibe were similar to those seen in Caucasian subjects.
- Hepatic Impairment: After a single 10-mg dose of ezetimibe, the mean AUC for total ezetimibe was increased approximately 1.7-fold in patients with mild hepatic impairment (Child-Pugh score 5 to 6), compared to healthy subjects. The mean AUC values for total ezetimibe and ezetimibe were increased approximately 3- to 4-fold and 5- to 6-fold, respectively, in patients with moderate (Child-Pugh score 7 to 9) or severe hepatic impairment (Child-Pugh score 10 to 15). In a 14-day, multiple-dose study (10 mg daily) in patients with moderate hepatic impairment, the mean AUC values for total ezetimibe and ezetimibe were increased approximately 4-fold on Day 1 and Day 14 compared to healthy subjects. Due to the unknown effects of the increased exposure to ezetimibe in patients with moderate or severe hepatic impairment, ezetimibe is not recommended in these patients.
- Renal Impairment: After a single 10-mg dose of ezetimibe in patients with severe renal disease (n=8; mean CrCl ≤30 mL/min/1.73 m2), the mean AUC values for total ezetimibe, ezetimibe-glucuronide, and ezetimibe were increased approximately 1.5-fold, compared to healthy subjects (n=9).
- Drug Interactions: Ezetimibe had no significant effect on a series of probe drugs (caffeine, dextromethorphan, tolbutamide, and IV midazolam) known to be metabolized by cytochrome P450 (1A2, 2D6, 2C8/9 and 3A4) in a "cocktail" study of twelve healthy adult males. This indicates that ezetimibe is neither an inhibitor nor an inducer of these cytochrome P450 isozymes, and it is unlikely that ezetimibe will affect the metabolism of drugs that are metabolized by these enzymes.
- Patients should be advised to adhere to their National Cholesterol Education Program (NCEP)-recommended diet, a regular exercise program, and periodic testing of a fasting lipid panel.
- Patients should be advised to discuss all medication, both prescription and over-the-counter, with their physician.
- Patients should be advised of the risk of myopathy.
- Patients should report promptly any unexplained muscle pain, tenderness or weakness.
- Patients should be advised that liver tests should be performed when ezetimibe is added to statin therapy and according to statin recommendations.
- Women of childbearing age should be advised to use an effective method of birth control to prevent pregnancy while using ezetimibe added to statin therapy.
- Patients should discuss future pregnancy plans with their physician and discuss when to stop combination ezetimibe and statin therapy if they are trying to conceive.
- Patients should be advised that if they become pregnant they should stop taking combination ezetimibe and statin therapy and call their health care professional.
- Women who are breastfeeding should be advised to not use ezetimibe added to statin therapy.
- Patients who have a lipid disorder and are breastfeeding should be advised to discuss the options with their healthcare professionals.
Indications
Dosing
(Adult):
(Pediatrics):
Renal Dosing
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





