Ceftriaxone (Rocephin): Drug Monograph
|
|---|
- To treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. Infections included the following:
- Lower respiratory tract infections caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenza, Haemophilus parainfluenzae, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Proteus mirabilis or Serratia marcescens.
- Acute bacterial otitis media caused by Streptococcus pneumoniae, Haemophilus influenza (including beta-lactamase producing strains) or Moraxella catarrhalis (incuding beta-lactamase producing strains).
- Skin and skin structure infections caused by Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Viridans group streptococci, Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii, Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter calcoaceticus, Bacteroides fragilis, or Peptostreptococcusspecies.
- Urinary tract infections (complicated and uncomplicated) caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Morganella morganii or Klebsiella pneumoniae.
- Uncomplicated gonorrhea (cervical/urethral and rectal) caused by Neisseria gonorrhoeae, including both penicillinase- and nonpenicillinase-producing strains, and pharyngeal gonorrhea caused by nonpenicillinase-producing strains of Neisseria gonorrhoeae.
- Pelvic inflammatory disease caused by Neisseria gonorrhoeae. No activity against Chlamydia trachomastis.
- Bacterial septicemia caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae or Enterobacter species.
- Bone and joint infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, or Enterobacter species.
- Intra-abdominal infections caused by Escherichia coli, Klebsiella pneumoniae, Bacteroides fragilis, Clostridium species or Peptostreptococcusspecies.
- Meningitis caused by Haemophilus influenza, Neisseria meningitids or Streptococcus pneumoniae.
- Surgical prophylaxis
- Cervicitis (from Gonococcal Infection):
- 250 mg IM x 1 dose. Note: can mix with 1% lidocaine for injection to reduce localized pain/discomfort)
- If Chlamydia trachomatis is suspected as well, then consider azithromycin 1 g orally x 1 or doxycycline 100 mg orally twice daily as well
- Cholecystitis:
- 1 - 2 g IV every 12 to 24 hrs x 4-7 days along with appropriate source control.
- Conjunctivitis
(Gonococcal; Complicated):
- 1 g IM x 1 dose
- Endocarditis
- Native Valve: 2 g IV once daily x 14 days +/- gentamicin for synergy
- Prosthetic Valve: 2 g IV once daily x 6 weeks (4 weeks for HACEK organisms) +/- gentamicin
- Prophylaxis for Dental Procedures: 1 g IV 30 - 60 min prior to procedure
- Epididymitis:
- 250 mg IM x 1 dose plus doxycycline 100 mg orally twice a day x 10 days
- Gonococcal Infections:
- Cervix, urethra, rectum, or pharynx: 250 mg IM x 1 dose with either azithromycin 1 g orally x 1 dose or doxycycline 100 mg orally twice a day for 7 days to cover against Chlamydia trachomatis
- Conjunctivitis (complicated): 1 g IM x 1 dose
- Disseminated: 1 g IV/IM once daily for at least 2 days and then consider switch to oral cefixime
- Epididymitis: 250 mg IM x 1 dose plus doxycycline 100 mg orally twice a day x 10 days
- Pelvic Inflammatory Disease (PID): 250 mg IM x 1 dose + doxycycline 100 mg orally twice daily x 14 days (unless pregnant then replace doxycycline with azithromycin 1 g orally weekly x 2) +/- metronidazole 500 mg orally twice a day x 14 days
- Intra-Abdominal
Infection:
- 1 - 2 g IV every 12 - 24 hrs x 4 - 7 days
- Give with metronidazole 500 mg IV every 6 hrs. Note: Can give first dose as 1 g.
- Lyme Disease:
- 2 g IV once daily x 14 - 28 days
- Mainly used when concerned about Lyme meningitis, otherwise other can consider doxycycline
- Meningitis:
- 1 - 2 g IV every 12 hrs x 10 - 14 days
- If empirically starting consider giving ceftriaxone 2 g IV every 12 hrs along with:
- Vancomycin 15 - 20 mg/kg IV every 8 - 12 hrs +/-
- Dexamethasone 0.15 mg/kg IV every 6 hrs x 2 - 4 days (starting before or with the first dose of antibiotic) +/-
- Ampicillin 2 g IV every 4 hrs (if concerned about Listeria such as in patients > 50 yrs of age, who are immunocompromised, or pregnant)
- Meningococcal Prophylaxis (High-Risk Contacts with Close Contact with Invasive Meningococcal Disease):
- 250 mg IM x 1 dose
- Pelvic Inflammatory Disease (PID):
- 250 mg IM x 1 dose + doxycycline 100 mg orally/IV twice daily x 14 days (unless pregnant then replace doxycycline with azithromycin 1 g orally weekly x 2) +/- metronidazole 500 mg orally twice a day x 14 days
- Pneumonia (Community Acquired; CAP):
- 1 g IV daily for at least 5-7 days plus addition of a macrolide such as clarithromycin or azithromycin to cover atypical organisms (Chlamydia pneumonia, Legionella pneumonia, Mycoplasma pneumonia).
- For patients admitted to the ICU or where there might be concern for resistant organisms consider starting dose at 2 g IV daily.
- Proctitis, Proctocolitis:
- 250 mg IM x 1 dose + doxycycline 100 mg orally/IV twice daily x 14 days (unless pregnant then replace doxycycline with azithromycin 1 g orally weekly x 2)
- Pyelonephritis:
- 1 - 2 g IV once daily until can tolerate oral antibiotics then switch over to an outpatient regimen
- Surgical Prophylaxis (Preoperative):
- 1 g IV x 1 given 30 minutes to 2 hours before start of surgery
- Typhoid Fever:
- 2 g IV once daily x 14 days
- Urethritis (from Gonococcal Infection):
- 250 mg IM x 1 dose. Note: can mix with 1% lidocaine for injection to reduce localized pain/discomfort)
- If Chlamydia trachomatis is suspected as well, then consider azithromycin 1 g orally x 1 or doxycycline 100 mg orally twice daily as well
- General Notes: May be administered intravenously or intramuscularly. Incompatible with calcium containing solutions including Lactated Ringers or TPN.
- Conjunctivitis (Gonococcal; Complicated):
- If < 45 kg: 50 mg/kg IV/IM x 1 dose (max dose of 1 g)
- If > 45 kg: 1 g IV/IM x 1 dose
- Endocarditis:
- Native Valve: 100 mg/kg IV/IM once daily x 2 - 4 weeks
- Prosthetic Valve: 100 mg/kg IV/IM once daily x 6 weeks +/- gentamicin
- Enterococcus faecalis: 50 mg/kg IV/IM every 12 hrs x at least 8 weeks + ampicillin
- Prophylaxis Against IE: 50 mg/kg IV/IM 30 - 60 min before procedure to max dose of 1 g.
- Epididymitis:
- If > 8 yrs of age (> 45 kg): 125 mg IM x 1 dose
- Epiglotitis:
- 50 - 100 mg/kg/day IM or IV x 2 - 14 days
- Gonococcal Infections:
- Disseminated: 50 mg/kg/dose IV/IM once daily x 7 days
- Lyme Disease:
- 75 - 100 mg/kg x 2 - 4 weeks (max dose of 2 g)
- Meningitis:
- 100 mg/kg IV loading dose, then 50 mg/kg IV every 12 hrs x 7 - 14 days
- Meningococcal Prophylaxis (High-Risk Contacts with Close Contact with Invasive Meningococcal Disease):
- If < 15 yrs of age: 125 mg IM x 1 dose
- If > 15 yrs of age: 250 mg IM x 1 dose
- Ophthalmia Neonatorum:
- Infants: 25 - 50 mg/kg x 1 dose (max of 125 mg)
- STD Prophylaxis After Sexual Assault:
- If < 45 kg: 125 mg IM x 1 dose + azithromycin + metronidazole
- If > 45 kg: 250 mg IM x 1 dose + azithromycin 1 g orally weekly x 2 +/- metronidazole 500 mg orally twice a day x 14 days
- Pneumonia (Community Acquired; CAP):
- 50-100 mg/kg/day IV daily or two divided doses for at least 5-7 days.
- Consider the addition of a macrolide such as clarithromycin or azithromycin to cover atypical organisms (Chlamydia pneumonia, Legionella pneumonia, Mycoplasma pneumonia).
- Consider the addition of vancomycin or clindamycin if concerned about MRSA pneumonia
- Surgical Prophylaxis (Preoperative):
- 50 - 75 mg/kg IV x 1 given 30 - 60 minutes before start of surgery
- Typhoid Fever:
- 75 - 80 mg/kg IV once daily x 5 - 14 days
- None
- Minimal removal by hemodialysis, peritoneal dialysis, or CVVHD. No supplemental doses or dose adjustments needed.
- Known allergy to the cephalosporin class of antibiotics
- Hyperbilirubinemic neonates (≤ 28 days)
- Hypersensitivity to cephalosporins, penicillins or other drugs
- Interaction with calcium-containing products: ceftriaxone must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site.
- Patients with history of gastrointestinal disease, especially colitis - prescribe with caution
- Prolonged use of ceftriaxone may result in overgrowth of nonsusceptible organisms
- Local reactions - pain, induration and tenderness, warmth, tightness or induration
- Hypersensitivity - rash
- Hematologic - eosinophilia, thrombocytosis, and leukopenia.
- Diarrhea, onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment
- Hepatic - elevations of SGOT or SGPT
- Renal - elevations of the BUN
- Hemolytic anemia
- Treatment should be symptomatic
- Drug concentration would not be reduced by hemodialysis or peritoneal dialysis
- Pregnancy: Pregnancy Category B
- Labor and Delivery: None
- Nursing Mothers: Low concentrations are excreted in human milk. Use caution when administering.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Safety and effectiveness in neonates, infants and pediatric patients have been established for the dosages described. Should not be administered to hyperbilirubinemic neonates, especially prematures.
- Geriatric Patients: No overall differences in safety or effectiveness were observed between geriatric and younger subjects. No dosage adjustments are necessary for geriatric patients with ceftriaxone dosages up to 2 grams per day.
-
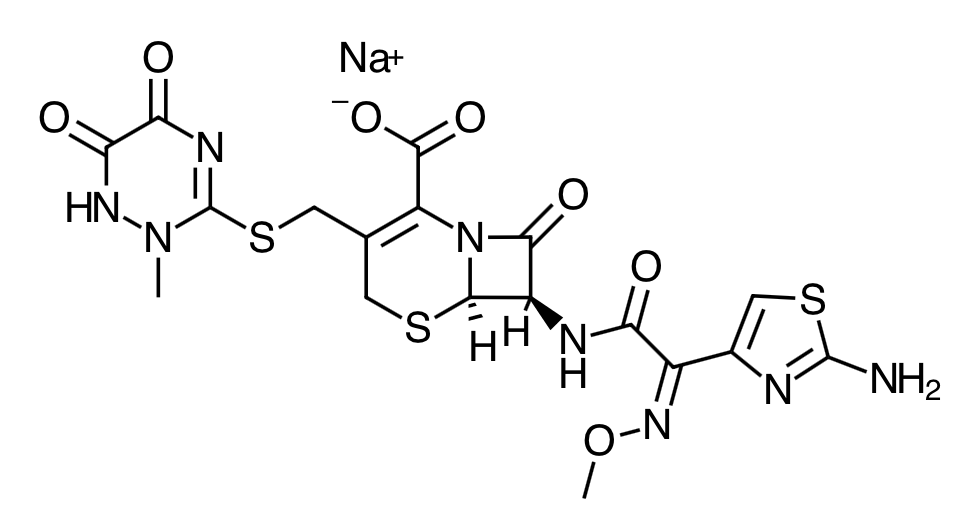
Scientific Name:
(6R,7R)-7-[2-(2- Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as- triazin-3-yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72 -(Z)- (O-methyloxime), disodium salt, sesquaterhydrate -
Empirical Formula:
C18H16N8Na2O7S3.3.5H2O -
Molecular Weight:
661.59 - Ceftriaxone is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis through inhibition of transpeptidase or penicillin binding protein. It also known to exhibit time-dependent killing.
- Ceftriaxone has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
- Absorption: Ceftriaxone was completely absorbed following IM administration with mean maximum plasma concentrations occurring between 2 and 3 hours post-dose.
- Volume of Distribution:5.78 to 13.5 L
- Protein Binding: Reversibly bound to human plasma proteins
- Distribution: Ceftriaxone crosses the blood placenta barrier
- Metabolism: None
- Elimination: Plasma clearance ranged from 0.58 to 1.45 L/hour and renal clearance from 0.32 to 0.73 L/hour. Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug and the remainder was secreted in the bile.
- Patients should be counseled that antibacterial drugs including ceftriaxone should only be used to treat bacterial infections. They do not treat viral infections, such as the common cold.
- Patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by ceftriaxone or other antibacterial drugs in the future. Patients should be counseled that diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued.
- Patients may develop watery and bloody stools (with or without stomach cramps and fever), even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Indications
Dosing (Adult)
General Notes: May be administered intravenously or intramuscularly and given in as a single dose as high as 2 g or as 2 divided doses. Total daily dose not to exceed 4 grams. Incompatible with calcium containing solutions including Lactated Ringers or TPN.
Dosing (Pediatric)
Renal Dosing
Contraindications
Warnings
Adverse Reactions
Overdose
Special Populations
Chemical Structure
Mechanism of Action
Pharmacokinetics
Counseling Points
Related Content
|
|---|
|
MESH Terms & Keywords
|
|---|
|

