Aclidinium Bromide (Tudorza Pressair): Drug Monograph
|
|---|
- Dispensed as a multi-dose device is a dry powder inhaler metering 400 mcg of Aclidinium bromide per actuation
- Not for acute COPD exacerbations
- Paradoxical bronchospasm with discontinuation
- Worsening of narrow-angle glaucoma
- Worsening
of urinary retention, especially in patients with BPH
- Immediate hypersensitivity reactions to milk proteins
- Anticholinergics - additive potential if concomitantly used with other anticholinergic medications. Avoid use with these drugs.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: The effect on labor and delivery is unknown.
- Nursing Mothers: Excretion of aclidinium into human milk is probable. Use with caution in nursing women.
- Renal Impairment: None.
- Hepatic Impairment: Not studied.
- Pediatric Patients: The safety and effectiveness has not been established.
- Geriatric Patients: No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects. Greater sensitivity of some older individuals cannot be ruled out, however no dosage adjustment is warranted.
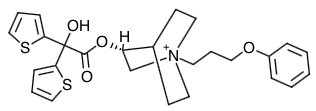
- Scientific Name: Azoniabicyclo[2.2.2]octane, 3-[(hydroxydi-2-thienylacetyl)oxy]-1-(3- phenoxypropyl)-, bromide, (3R)-
- Empirical Formula: C26H30NO4S2Br
- Molecular Weight: 564.56
- Aclidinium bromide is a long-acting inhaled antimuscarinic agent with similar affinity to the subtypes of muscarinic receptors M1 to M5.
- In the airways, it exhibits pharmacological effects topically through a competitive and reversible inhibition of M3 receptor at the smooth muscle of the bronchioles thereby leading to smooth muscle dilation (i.e., bronchodilation).
-
Cardiovascular Effects: No effect on QT interval was seen in a study using 200 mcg and 800 mcg aclidinium bromide in healthy volunteers once daily for 3 days.
- Absorption: Absolute bioavailability is ~ 6% in healthy volunteers with peak steady state plasma levels were observed within 10 minutes after inhalation.
- Volume of Distribution: ~ 300 L following intravenous (IV) administration of 400 mcg in humans.
- Metabolism: Primarily hydrolysis by esterases to its inactive alcohol and dithienylglycolic acid derivatives, which do not bind to muscarinic receptors.
- Elimination: Clearance was ~ 170 L/h after an IV doses of aclidinium bromide in young healthy volunteers with an inter-individual variability of 36%. After dry powder inhalation, urinary excretion is ~ 0.09%.
- Half-Life: 5 to 8 hours
- Drug Interactions: No effect on the CYP450 enzymes or cell membrane transporters of drug distribution
- Elderly Patients: No clinically significant differences in have been seen with this patient population and no dosage adjustment is necessary.
- Renal Impairment: Based on pharmacokinetic studies, no dose adjustment is necessary in renally impaired patients.
- Hepatic Impairment: This has not been studied but changes would be expected.
- The inhaler should be stored inside the sealed pouch and only opened immediately before use.
- Instruct patients that aclidinium bromide is a twice daily maintenance bronchodilator and should not be used for immediate relief of breathing problems (i.e., as a rescue medication).
- Inform patients that if they miss a dose, they should take their next dose at the usual time; they should not take 2 doses at one time.
- Inform patients that the drug can cause paradoxical bronchospasm.
- Advise patients that if paradoxical bronchospasm occurs, patients should discontinue the drug.
- Inform patients that eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema may be signs of acute narrow-angle glaucoma.
- Inform patients to consult a physician immediately should any of these signs and symptoms develop.
- Advise patients that miotic eyedrops alone are not considered to be effective treatment.
- Inform patients that care must be taken not to allow the powder to enter into the eyes as this may cause blurring of vision and pupil dilation.
- Inform patients that difficulty passing urine and dysuria may be symptoms of new or worsening prostatic hyperplasia or bladder outlet obstruction.
- Patients should be instructed to consult a physician immediately should any of these signs or symptoms develop.
Dosage Forms
Warnings
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
Paradoxical bronchospasm
Visual effects
Urinary retention
MESH Terms & Keywords
|
|---|
|





