It
is well known that patients with type 2 diabetes mellitus (T2DM) are
characterized as having insulin resistance, a decrease in insulin mediated
glucose uptake by peripheral tissues (despite elevated insulin levels) and
excessive basal rates of hepatic gluconeogenesis.1,2 An impairment in
peripheral glucose uptake and suppression of gluconeogenesis both contribute to
worsening postprandial (post-meal) hyperglycemia whereas excessive basal rates
of hepatic gluconeogenesis primarily contributes to the worsening of fasting
glucose levels. To date, the biguanide class of medications primarily
suppresses the excessive basal rates of gluconeogenesis which includes primarily
metformin (Glucophage).3,4 The other biguanide, phenformin (Azucaps,
Insoral, Fenformin), is no longer FDA approved in the United States because of
unacceptable rates of lactic acidosis but can still be used and/or purchased by
clinicians/patients in other countries.5
Why
do type 2 diabetics have excessive rates in basal hepatic glucose production?
Normally, the breakdown of glycogen and gluconeogenesis in the liver are both
in part regulated by the presence of insulin and have a direct impact on
fasting blood glucose levels.1 However, with T2DM being in a state of
insulin resistance, the ability of insulin to activate protein phosphatases,
which dephosphorylates glycogen phosphorylase a and glycogen synthase b that
shut off glycogen breakdown, is decreased, thereby allowing a
greater amount of glycogen to be converted to Glucose 1-phosphate. In
addition, the state of insulin resistance may also not be as
efficient at regulating or "slowing down" the two critical steps in
gluconeogenesis that also puts more glucose into the blood. The first
enzyme lacking regulation in insulin resistance is phosphoenolpyruvate
carboxykinase ((PEPCK); which converts oxaloacetate to phosphoenolpyruvate) and
the second is a reduction in the amount of fructose 2,6-bisphosphate (F-2,6-P)
produced by insulin which can then inhibit the enzyme fructose
1,6-bisphosphatase. All of the above abnormally regulated processes lead
to a greater amount of glucose 6-phospate that can then be converted back to
glucose in the blood via glucose 6-phosphatase (an enzyme only found in the
liver).
How
then does metformin affect one or both of these abnormally regulated processes
in hepatic gluconeogenesis?
Metformin's primary benefit in T2DM has been in its ability to "slow
down" the accelerated basal rates of hepatic gluconeogenesis without an
apparent effect on lactate turnover for gluconeogenesis or increases in insulin
secretion.3,4 Metformin does this by decreasing the amount of enzymes
phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (see
figure).6
How
does it do this?
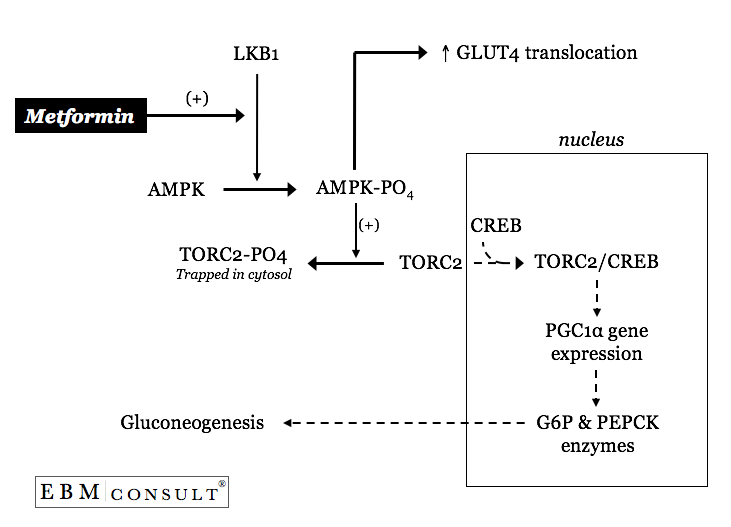
Metformin can activate an upstream primary kinase called LKB1 thereby resulting
in the phosphorylation of AMP-activated protein kinase (AMPK).7
The phosphorylated AMPK will then result in the cytosolic sequestering of the
CREB transcription factor named transducer of regulated CREB
activity 2 (TORC2).7 With TORC2 now trapped in the
cytosol of the hepatocyte (liver cell) CREB within the nucleus is now notas
efficient at transcribing a transcriptional cofactor named peroxisome
proliferator-activated receptor-g co-activator 1a (PGC1a).7 With lower
amounts of PGC1a there is less transcriptional activation of glucose
6-phosphatase and PEPCK thereby leading to a "slowing down" of the
excessive basal rates of hepatic gluconeogeneis.7 Interestingly,
metformin's activation of AMPK also contributes to overall glucose control by
increasing AMPK mediated increases in translocation of GLUT-4 transporters in
muscle.8
Therefore,
metformin improves fasting blood sugars by slowing down the
"excessive" basal hepatic gluconeogenesis without significant changes
in insulin levels that would be known to cause hypoglycemia.3,4,9 The
average reductions in fasting blood glucose levels and hemoglobin A1c while on
metformin are approximately 44-53 mg/dL (2.4-2.9 mmol/L)and 1.4-2%, respectively.3,4,9
References:
- Monnier L, Colette C, Owens DR.
Type 2 diabetes: a well-characterised but suboptimally controlled
disease. Can we bridge the divide? Diabetes Metab. 2008;34(3):207-216.
- Leiberman M, Marks AD, eds. Mark's Basic Medical Biochemistry
A Clinical Approach. 3rd
Ed. Philadelphia, PA: Lippincott
Williams & Wilkins; 2009:479-566.
- Bristol-Myers Squibb Co.
Glucophage (metformin hydrochloride) package insert. Princeton, NJ;
August 2008. Link obtained on
11/24/2008: Package Insert
- Cusi K, Consoli A, DeFronzo RA.
Metabolic effects of metformin on glucose and lactate metabolism in
noninsulin-dependent diabetes mellitus.
J Clin Endocrinol Metab 1996;81:4059-4067.
- Kumar A, Nugent K, Kalakunja A, Pirtle F. Severe acidosis in a patient with type 2
diabetes mellitus, hypertension and renal failure. CHEST
2003;123:1726-1729.
- Mithieux G, Guignot L, Bordet J, Wiernsperger N. Intrahepatic mechanisms underlying the effect
of metformin in decreasing basal glucose production in rats fed a high fat
diet. Diabetes 2002;51:139-143.
- Shaw RJ, Lamia KA, Vasquez D et al. The kinase LKB1 mediates
glucose homeostasis in liver and therapeutics effects of metformin. Science
2005;310(5754):1642-1646.
- Yamaguchi S. Katahira H, Ozawa S et al. Activators of
AMP-activated protein kinase enhance GLUT4 translocation and its glucose
transport activity in 3T3-LI adipocytes. Am J Physiol Endocrinol Metab 2005;289(4):E643-E649.
- DeFronzo RA, Goodman AM,
The Multicenter Metformin Study Group.
Efficacy of metformin in patients with non-insulin-dependent diabetes
mellitus. N Engl J Med 1995;333:541-549.
- Nathan DM, Buse JB, Davidson MB et al.
Management of hyperglycemia in type 2 diabetes: a consensus algorithm
for the initiation and adjustment of therapy: a consensus statement from the
American Diabetes Association and the European Association for the Study of
Diabetes. Diabetes Care 2006;29(8):1963-1972.


