Avanafil (Stendra): Drug Monograph
|
|---|
- General Notes:
- Take no more than once a day with or without food.
- Do not use with strong CYP3A4 inhibitors.
- If taking a moderate CYP3A4 inhibitor, the dose should be no more than 50 mg in a 24-hour period.
- Erectile Dysfunction:
- 100 mg taken approximately 30 minutes before sexual activity
- In patients on stable alpha-blocker therapy: the recommended starting dose is 50 mg.
- In patients also taking moderate inhibitors of CYP3A4: Limit to 50 mg once a day.
- In patients also taking strong CYP3A4 inhibitors: Avoid use of avanafil.
- None
- Administration to patients using any form of organic nitrate is contraindicated.
- Hypersensitivity to any component of the tablet.
- Patients should not use if sexual activity is inadvisable due to cardiovascular status or any other reason.
- Use with alpha-blockers, other antihypertensives, or substantial amounts of alcohol (greater than 3 units) may lead to hypotension.
- Erection lasting > 4 hours - seek emergency treatment.
- Sudden loss of vision in one or both eyes as this could be a sign of Non Arteritic Ischemic Optic Neuropathy (NAION). Stop treatment and seek medical attention.
- Sudden decrease or loss of hearing - stop treatment and seek prompt medical attention.
- Alcohol - Consuming > 3 units may increase the potential for orthostatic signs and symptoms
- Combination with other PDE5 inhibitors or erectile dysfunction therapies is not recommended
- Standard supportive measures should be adopted as required.
- Renal dialysis is not expected to accelerate clearance.
- Can potentiate the hypotensive effect of nitrates, alpha-blockers, antihypertensives, and alcohol.
- CYP3A4 Substrate: Major
- Caution with CYP3A4 Inhibitors: (e.g., ketoconazole, ritonavir, erythromycin) increase avanafil exposure.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: None
- Renal Impairment: No dose adjustment necessary for patients with mild to moderate impairment. Severe impairment: do not use.
- Hepatic Impairment: No dose adjustment necessary for patients with mild to moderate impairment. Severe impairment: do not use.
- Pediatric Patients: Not indicated for use in pediatric patients. Safety and efficacy in patients below the age of 18 years has not been established.
- Geriatric Patients: No dose adjustment is warranted based on age alone. A greater sensitivity to medication in some older individuals should be considered.
-
Scientific Name: (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2- pyrimidinylmethyl)-5-pyrimidinecarboxamide
-
Empirical Formula: C23H26ClN7O3
-
Molecular Weight: 483.95
- The mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation, which then activates the enzyme guanylate cyclase leading to increased levels of cGMP, producing smooth muscle relaxation in the corpus cavernosum and blood flow that leads to an erection.
- Avanafil is also more potent on PDE5 than on other known phosphodiesterases (greater than 100-fold for PDE6; greater than 1,000-fold for PDE4, PDE8 and PDE10; greater than 5,000-fold for PDE2 and PDE7; greater than 10,000-fold for PDE1, PDE3, PDE9, and PDE11). Avanafil is greater than 100-fold more potent for PDE5 than PDE6, which is found in the retina and is responsible for phototransduction.
- Effects on Erectile Response:
- In a single-blind, placebo-controlled, single-dose trial of 82 patients with either organic and/or psychogenic ED, visual sexual stimulation resulted in improved erections after avanafil administration compared to placebo, as assessed by an objective measurement of hardness and duration of erections (RigiScan®). Efficacy was assessed by RigiScan at discrete time intervals ranging from 20-40 minutes after dosing to 100-120 minutes after dosing.
- Effects on Blood Pressure:
- Single oral doses of avanafil (200 mg) administered to healthy male volunteers resulted in mean changes from baseline in systolic/diastolic blood pressure of -5.3/-3.7 mmHg at 1 hour after dosing, compared to mean changes from baseline in the placebo group of 2.7/-0.4 mmHg. The reductions in systolic/diastolic blood pressure at 1 hour after dosing of avanafil 200 mg compared to placebo were 8.0/3.3 mmHg.
- Effects on Cardiac Electrophysiology:
- The effect of single 100 or 800 mg doses of avanafil on the QT interval were evaluated in a randomized, double-blind, placebo, and active (moxifloxacin) -controlled crossover study in 52 healthy male subjects aged 18 to 45 years. There were no significant effects of the 100 mg dose. The mean QTc (Fridericia QT correction) for avanafil 800 mg, relative to placebo was 9.4 milliseconds (two-sided 90% CI=7.2, 11.6). An 800 mg dose of avanafil (4 times the highest recommended dose) was chosen because this dose yields exposures greater than those observed upon co-administration of avanafil with strong CYP3A4 inhibitors. A double blind, randomized, placebo- and active-controlled (moxifloxacin), thorough QT/QTc trial of avanafil (100 and 800 mg) in healthy male subjects demonstrated that avanafil did not cause any significant changes in QTc interval or ventricular repolarization.
- Effects on Blood Pressure When Administered with Nitrates:
- In a clinical pharmacology trial, a single dose of avanafil 200 mg was shown to potentiate the hypotensive effect of nitrates. The use of avanafil in patients taking any form of nitrates is contraindicated.
- A trial was conducted to assess the degree of interaction between nitroglycerin and avanafil, should nitroglycerin be required in an emergency situation after avanafil was taken. This was a single-center, double blind, randomized, 3-way crossover trial of healthy males from 30 to 60 years of age. Subjects were divided among 5 trial groups with the trial group being determined by the time interval between treatment with trial drug and glyceryl trinitrate administration. Subjects were assigned to trial groups sequentially and hemodynamic results from the previous group were reviewed for serious adverse events (SAEs) before the next group received treatment. Each subject was dosed with all 3 study drugs (avanafil 200 mg, sildenafil citrate 100 mg, and placebo) in random order. Subjects were administered a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre-specified time points, following their dose of trial drug (0.5, 1, 4, 8 or 12 hours). Overall, 14 (15%) subjects treated with placebo and 28 (28%) subjects treated with avanafil, had clinically significant decreases in standing SBP, defined as greater than or equal to 30 mmHg decrease in SBP, after glyceryl trinitrate administration.
- Like other PDE5 inhibitors, avanafil administration with nitrates is contraindicated. In a patient who has taken avanafil, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of avanafil before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring.
- Effects on Blood Pressure When Administered with Alpha-Blockers:
- A single-center, randomized, double-blinded, placebo-controlled, two-period crossover trial was conducted to investigate the potential interaction of avanafil with alpha-blocker agents in healthy male subjects which consisted of two cohorts:
- Cohort A (N=24): Subjects received oral doses of doxazosin once daily in the morning at 1 mg for 1 day (Day 1), 2 mg for 2 days (Days2-3),4 mg for 4 days (Days 4-7), and 8 mg for 11 days (Days 8-18). On Days 15 and 19, the subjects also received a single oral dose of either 200 mg avanafil or placebo, according to the treatment randomization code. The avanafil or placebo doses were administered 1.3 hours after the doxazosin administration on Days 15 and 18. The co-administration was designed so that doxazosin (Tmax ~2 hours) and avanafil (Tmax ~0.7 hours) would reach their peak plasma concentrations at the same time.
- Cohort B (N=24): Subjects received 0.4 mg daily oral doses of tamsulosin in the morning for 11 consecutive days (Days 1 - 11). On Days 8 and 11, the subjects also received a single oral dose of either 200 mg STENDRA or placebo, according to the treatment randomization code. The STENDRA or placebo doses were administered 3.3 hours after the tamsulosin administration on Days 8 and 11. The co-administration was designed so that tamsulosin (Tmax ~4 hours) and avanafil (Tmax ~0.7 hours) would reach their peak plasma concentrations at the same time.
- Supine and sitting BP and pulse rate measurements were recorded before and after avanafil or placebo dosing. A total of seven subjects in Cohort A (doxazosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Three subjects experienced standing SBP values less than 85 mmHg. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following avanafil. Two subjects experienced standing DBP values less than 45 mmHg following avanafil. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following avanafil. One subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
- A total of five subjects in Cohort B (tamsulosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Two subjects experienced standing SBP values less than 85 mmHg following avanafil. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following avanafil. Two subjects experienced standing DBP values less than 45 mmHg following avanafil. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following avanafil; one subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
- Effects on Blood Pressure When Administered with Enalapril:
- A trial was conducted to assess the interaction of enalapril (20 mg daily) and avanafil 200 mg. Single doses of 200 mg avanafil co-administered with enalapril caused a mean maximum decrease in supine systolic/diastolic blood pressure of 1.8/3.5 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm.
- Effects on Blood Pressure When Administered with Amlodipine:
- A trial was conducted to assess the interaction of amlodipine (5 mg daily) and avanafil 200 mg. Single doses of 200 mg avanafil co-administered with amlodipine caused a mean maximum decrease in supine systolic blood pressure of 1.2 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm; the mean maximum decrease in diastolic blood pressure was less than that observed in the placebo group. There was no effect of avanafil on amlodipine plasma concentrations. Concomitant amlodipine was associated with 22% and 70% increases in avanafil Cmax and AUC, respectively.
- Effects on Blood Pressure When Administered with Alcohol:
- Alcohol and PDE5 inhibitors, including avanafil, are mild systemic vasodilators. The interaction of avanafil with alcohol was evaluated in a clinical pharmacology trial. Alcohol was administered at a dose of 0.5 g/kg, which is equivalent to approximately 3 ounces of 80-proof vodka in a 70-kg male, and avanafil was administered at a dose of 200 mg. All patients consumed the entire alcohol dose within 15 minutes of starting. Blood alcohol levels of 0.057% were confirmed. There were no reports of orthostatic hypotension or dizziness. Additional maximum supine systolic/diastolic blood pressure decreases of 3.5/4.5 mm Hg and additional maximum pulse rate increase of 9.3 bpm were observed when avanafil was taken with alcohol compared to alcohol alone. Avanafil did not affect alcohol plasma concentrations.
- Effects on Semen:
- A single 200 mg dose of avanafil had no acute effect on sperm motility or sperm morphology in a group of healthy male subjects. The effect of avanafil on human spermatogenesis is unknown.
- Effects on Vision:
- Single oral doses of Type 5 phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina.
- Absorption:
- Rapid oral absorption with a median Tmax of 30 to 45 minutes in the fasted state. While a high fat meal can reduce the rate of absorption, the changes are not significant. Therefore, avanafil may be administered with or without food.
- Distribution:
- 99% bound to plasma proteins and is independent of total drug concentrations, age, renal and hepatic function.
- Metabolism:
- Primarily metabolized by the CYP3A4 enzyme and to a minor extent by CYP2C isoform.
- Elimination:
- Excreted as metabolites predominantly in the feces (approximately 62%) and to a lesser extent in the urine (approximately 21%).
- Terminal elimination half-life of about 5 hours.
- Renal Impairment: The pharmacokinetics of a single 200 mg avanafil administered to fourteen healthy elderly male volunteers (65-80 years) and eighteen healthy younger male volunteers (18-43 years of age) were compared. AUC0-inf decreased by 2.9% and Cmax increased by 2.8% in patients with mild renal impairment, compared to healthy volunteers with normal renal function. AUC0-inf increased by 9.1% and Cmax decreased by 2.8% in patients with moderate renal impairment, compared to healthy volunteers with normal renal function. There is no data available for subjects with severe renal insufficiency or end-stage renal disease on hemodialysis.
- Hepatic Impairment: The pharmacokinetics of a single 200 mg avanafil administered to eight patients with mild hepatic impairment (Child-Pugh A) and eight patients with moderate hepatic impairment (Child-Pugh B) were evaluated. AUC0-inf increased by 3.8% and Cmax decreased by 2.7% in patients with mild hepatic impairment, compared to healthy volunteers with normal hepatic function. AUC0-inf increased by 11.2% and Cmax decreased by 51% in patients with moderate hepatic impairment, compared to healthy volunteers with normal hepatic function. There is no data available for subjects with severe hepatic impairment (Child-Pugh Class C).
- Drug Interactions:
- Strong CYP3A4 Inhibitors: Co-administration with the strong CYP3A4 inhibitor ketoconazole can cause an approximate 13-fold increase in AUC0-inf and 3.1-fold increase in Cmax. Co-administration with the strong CYP3A4 inhibitor ritonavir can cause an approximate 13-fold increase in AUC0-inf and 2.4-fold increase in Cmax.
- Moderate CYP3A4 Inhibitors: Co-administration with the moderate CYP3A4 inhibitor erythromycin can cause an approximate 3.6-fold increase in AUC0-inf and 2.0-fold increase in Cmax.
- Effect of Avanafil on Other Drugs
- Warfarin:
- The effect of avanafil on warfarin pharmacokinetics and pharmacodynamics was evaluated in a double blind, randomized, placebo- controlled, two-way crossover study. Twenty-four healthy male volunteers were randomized to receive either 200 mg avanafil or matching placebo for 9 days. On Day 3 of each period, volunteers received a single 25 mg warfarin. Pharmacokinetics of R- and S- warfarin, PT, and INR prior to warfarin dosing and up to 168 hours after warfarin administration were compared. Platelet aggregation prior to warfarin dosing and up to 24 hours after warfarin administration were compared. PT, INR, and platelet aggregation did not change with avanafil administration: 23.1 sec, 2.2, and 75.5%, respectively. Co-administration with avanafil resulted in an approximate 1.6% increase in AUC0-inf and 5.2% decrease in Cmax of S-warfarin.
- Patients should be counseled that concomitant use with organic nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
- Physicians should discuss with patients the appropriate action in the event that they experience angina chest pain requiring nitroglycerin following intake of avanafil. In such a patient where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of v=avanafil before nitrate administration is considered.
- Nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring.
- Advise patients who experience angina chest pain after taking avanafil to seek immediate medical attention.
- Patients should be advised about the potential cardiac risk of sexual activity in patients with preexisting cardiovascular risk factors and to refrain from further sexual activity and seek immediate medical attention if they experience symptoms upon initiation of sexual activity.
- Advise patients of the potential for avanafil to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications.
- Advise patients to contact the prescribing physician if new medications that may interact with avanafil are prescribed by another health care provider.
- Advise patients who have an erection lasting greater than 4 hours, whether painful or not, to seek emergency medical attention.
- Patients should be advised to stop use of all PDE5 inhibitors, including avanafil, and seek medical attention in the event of a sudden los of vision in one or both eyes.
- Advise patients of the increased risk of non-arteritic anterior ischemic optic neuropathy (NAION) in individuals who have already experienced NAION in one eye, including whether such individuals could be adversely affected by use of vasodilators, such as PDE5 inhibitors.
- Advise patients to stop taking avanafil and seek prompt medical attention in the event of sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness.
- Advise patients that both alcohol and avanafil act as mild vasodilators. Substantial consumption of alcohol (e.g., greater than 3 units) in combination with avanafil can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache.
- The use of avanafil offers no protection against sexually transmitted diseases. Physicians should consider counseling patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV).
- Physicians should discuss with patients the appropriate use of avanafil and its anticipated benefits.
- It should be explained that sexual stimulation is required for an erection to occur after taking avanafil.
- Avanafil should be taken approximately 30 minutes before initiating sexual activity.
- Patients should be informed that the recommended starting dose of avanafil is 100 mg and the dose may be increased to a maximum recommended dose of 200 mg or decreased to 50 mg based on efficacy and tolerability.
- Patients should be advised to contact their health care provider for dose modification.
- Patients should tell their health care provider about all the medicines they take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Dosing
(Adult)
(Pediatrics)
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Chemical Structure
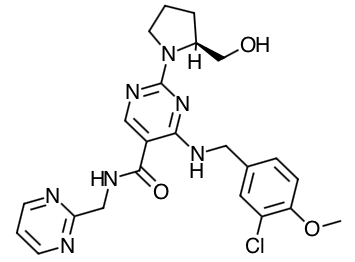
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|





