Acyclovir (Sitavig): Drug Monograph
|
|---|
- Application of one 50 mg buccal tablet as a single dose
- Known hypersensitivity to acyclovir, milk protein concentrate, or any other component of the product.
- Pregnancy: Pregnancy Category B
- Labor and Delivery: Should not be administered during labor and delivery, as there is no experience with acyclovir.
- Nursing Mothers: It is not known whether topically applied acyclovir is excreted in breast milk. Caution should be exercised when administered to a nursing woman.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Safety and effectiveness have not been established. Use in younger children is not recommended due to potential risk of choking.
- Geriatric Patients: Incomplete study data on this age group.
- It is not known whether topically applied acyclovir is excreted in breast milk. Caution should be exercised when administered to a nursing woman.
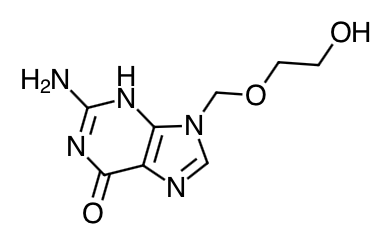
- Scientific Name: 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H purin-6-one
- Empirical Formula: C8H11N5O3
- Molecular Weight: 225
- Absorption: Salivary: Single dose application of 50 mg of acyclovir to the buccal mucosa in 12 healthy volunteers provided mean maximum salivary concentrations of 440 ?g/mL 8 hours after application of the tablet. The pharmacokinetic parameters of acyclovir in the saliva of healthy volunteers following application of a single 50 mg tablet in healthy volunteers are:
- AUC 0-24h (mcg.h/mL) - Mean ±SD (Min-Max)= 2900±2400 (849-9450)
- Cmax (mcg/mL) - Mean ±SD (Min-Max) = 440±241 (149-959)
- Tmax (hour) - Mean ±SD (Min-Max)= 7.95±4.08 (3.07-18.05)
- Distribution: Acyclovir is metabolized to 9-[(carboxymethoxy)methyl]guanine (CMMG) and 8 hydroxy-acyclovir (8-OH-ACV) by oxidation and hydroxylation.
- Elimination: Acyclovir is primarily excreted unchanged through the kidneys.
- Microbiology:
- Mechanism of Action: Acyclovir is a synthetic purine nucleoside that is phosphorylated intracellularly by the viral encoded thymidine kinase (TK) of HSV into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In a biochemical reaction, acyclovir triphosphate inhibits replication of herpes viral DNA by competing with nucleotides for binding to the viral DNA polymerase and by incorporation into and termination of the growing viral DNA chain. The cellular thymidine kinase of normal, uninfected cells does not use acyclovir effectively as a substrate, hence toxicity to mammalian host cells is low.
- Antiviral Activity: The quantitative relationship between the cell culture susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque-reduction assays on Vero cells, the median EC50 value of acyclovir against clinical herpes virus isolates (subjects receiving placebo) was 1.3 ?M (range: <0.56 to 3.3 ?M).
- Drug Resistance: Resistance of HSV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV with reduced susceptibility to acyclovir have been recovered from immunocompromised subjects, especially with advanced HIV infection. While most of the acyclovir-resistant mutant isolates from immunocompromised subjects thus far have been found to be TK-deficient, other mutant isolates involving the viral TK gene (TK partial and TK altered) or DNA polymerase have been identified. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in immunocompromised subjects who show poor clinical response during therapy.
- Patients should be informed that acyclovir is not a cure for cold sores.
- Patients should read the Instructions for Use that comes with acyclovir before starting the treatment. They should talk to their doctor or pharmacist if they have any questions.
- Patients should tell their health care provider about all the medicines they take, including prescription or over-the-counter medicines, vitamins, or herbal supplements.
- Patients should tell their health care provider if they are pregnant or plan to become pregnant and if they are breastfeeding or plan to breastfeed.
- The medication should be applied to the area of the upper gum above the incisor tooth. The tablets should not be crushed, sucked, chewed, or swallowed. If it comes out before 6 hours have gone by, reapply it. If this does not work, then a new tablet should be applied. It should not be applied to the inside of the lip or cheek.
- Tablet should be applied on the same side of the mouth as the herpes labialis symptoms.
- Advise the patient if they swallow the tablet within the first 6 hours of applying it, drink a glass of water and place a new tablet onto the upper gum.
- Acyclovir (Sitavig). Product Insert. Farmea. Angers, France. 2013. Acyclovir Product Page
General Notes: Acyclovir should be applied within one hour after the onset of prodromal symptoms and before the appearance of any signs of herpes labialis. Do not crush, chew, such or swallow tablets. Tablet should be placed to the upper gum just above the incisor tooth (canine fossa) and held in place with a slight pressure over the upper lip for 30 seconds to ensure adhesion. Tablet should be applied on the same side of the mouth as the herpes labialis symptoms. Food and drink can be taken normally when acyclovir is in place. Avoid any situations which may interfere with adhesion of the tablet such as chewing gum, touching or pressing the tablet after placement, wearing upper denture, and brushing teeth. Drink plenty of liquids in the case of dry mouth. If acyclovir does not adhere or falls off within the first 6 hours, the same tablet should be repositioned immediately. If the tablet cannot be repositioned, a new tablet should be placed. If the tablet is swallowed within the first 6 hours, the patient should drink a glass of water and a new tablet should be applied. Acyclovir does not need to be reapplied if the tablet falls out or is swallowed after the first 6 hours.
In the Phase 3 study, the levels of acyclovir in saliva were measured within 24 micrograms per mL) and were within the range of those observed in the PK study in healthy volunteers. In healthy volunteers, the median duration of buccal adhesion was 14 hours following application of a single acyclovir 50 mg tablet. Plasma concentrations of acyclovir were measured in 12 healthy volunteers after a single- dose application of SITAVIG 50 mg buccal tablet. Acyclovir concentrations had a delayed appearance (undetectable at 5 hours) and were below the concentrations required for antiviral activity (range: 17.5 to 55.3 nanogram per mL).
MESH Terms & Keywords
|
|---|
|





