Nicardipine (Cardene): Drug Monograph
|
|---|
- Injection: Short-term treatment of hypertension when oral therapy is not feasible or not desirable
- Oral treatment: treatment of hypertension alone or in combination with other antihypertensives
- General Notes:
- Injection: For intravenous use only. Administer by a central line or through a large peripheral vein; change infusion site every 12 hours if administered via peripheral vein. No further dilution is required in premixed solution. Do not combine with any product in the same IV line or premixed container; do not add supplementary medication to the bag.
- Ampules: administer by slow continuous infusion at a concentration of 0.1 mg/mL. Dilute each ampule (25 mg) with 240 mL of compatible IV fluid (yielding 250 mL of solution at a concentration of 0.1 mg/mL); diluted solution is stable for 24 hours at room temperature.
- Acute Hypertension (patients needing IV therapy):
- 5 mg/hr by IV infusion
- May increase by 2.5 mg/hr every 5 minutes (for rapid titration) to 15 minutes (for gradual titration) if desired blood pressure reduction is not achieved
- Maximum: 15 mg/hr
- Decrease infusion rate to 3 mg/hr after BP goal is achieved with rapid titration
- IV dosage as a substitute for oral nicardipine therapy:
- 20 mg by mouth every 8 hours = 0.5 mg/hr IV infusion
- 30 mg by mouth every 9 hours = 1.2 mg/hr IV infusion
- 40 mg by mouth every 8 hours = 2.2 mg/hr IV infusion
- Transition to oral nicardipine:
- Give 1 dose 1 hour prior to discontinuation of infusion
- Transfer to oral antihypertensive other than nicardipine:
- Initiate therapy upon discontinuation of IV nicardipine
- Hypertension, oral treatment:
- 30 mg by mouth twice daily
- Effective dose range: 30-60 mg twice daily
- Currently on nicardipine immediate-release (IR): titrate with sustained-release starting at current total daily dose of IR: reexamine adequacy of blood pressure control
- None
- Impaired function/reduced hepatic blood flow:
- Consider lower dosages and titrate slowly
- Injection: 2.5 mg/mL [10 mL, ampule]; 0.1 mg/mL, 0.2 mg/mL [200 mL, premixed solution]
- Capsules, sustained-release: 30 mg, 60 mg
- Excessive pharmacodynamic effects - close monitoring of blood pressure and heart rate is required. Nicardipine may occasionally produce symptomatic hypotension or tachycardia. Avoid systemic hypotension when administering the drug to patients who have sustained an acute cerebral infarction or hemorrhage.
- Angina, heart failure, impaired hepatic function, or renal impairment - closely monitor patient response
- Venous thrombosis, phlebitis, and vascular impairment - to reduce the possibility of these occurring, do not use small veins, such as those on the dorsum of the hand or wrist. Exercise extreme care to avoid intra-arterial administration or extravasation.
- Peripheral venous irritation - to minimize this risk, change the site of infusion of the injection every 12 hours.
- Symptoms may include marked hypotension, bradycardia, palpitations, flushing drowsiness, confusion and slurred speech. A lethal overdose may cause systemic hypotension, bradycardia (following initial tachycardia) and progressive atrioventricular conduction block.
- Implement standard measures including monitoring of cardiac and respiratory functions. Position the patient so as to avoid cerebral anoxia. Use vasopressors for patients exhibiting profound hypotension.
- CYP450 Enzymes: CYP3A4 substrate (major), minor substrate of CYP1A2, 2C9, 2D6, 2E1
- Transporters: P-gp (substrate)
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: Nicardipine is minimally excreted into human milk. Consider the possibility of infant exposure when using nicardipine in nursing mothers.
- Renal Impairment: Patients with moderate impairment have a significantly lower systemic clearance and higher area under the curve (AUC). Titrate gradually in such patients.
- Hepatic Impairment: Since nicardipine is metabolized in the liver, consider lower dosages and closely monitor responses in patients with impaired function or reduced hepatic blood flow.
- Pediatric Patients: Safety and efficacy in patients under the age of 18 have not been established.
- Geriatric Patients: In general, use low initial doses in elderly patients, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
- Nicardipine is minimally excreted into human milk. Consider the possibility of infant exposure when using nicardipine in nursing mothers.
-
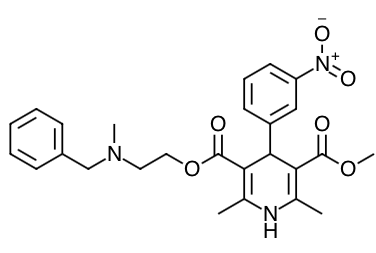
Scientific Name: (±)-2-(benzyl-methyl amino) ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride
-
Empirical Formula: C26H29N3O6HCl
-
Molecular Weight: 515.99
- Nicardipine inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle without changing serum calcium concentrations, however, the effects are more selective to vascular smooth muscle than cardiac muscle.
- In animal models, nicardipine produced relaxation of coronary vascular smooth muscle at drug levels, which cause little or no negative inotropic effect.
-
Hemodynamics:
-
Nicardipine I.V. produces significant decreases in systemic vascular resistance.
-
An increase in heart rate is a normal response to vasodilation and decrease in blood pressure; in some patients these increases in heart rate may be pronounced.
-
Hemodynamic studies following intravenous dosing in patients with coronary artery disease and normal or moderately abnormal left ventricular function have shown significant increases in ejection fraction and cardiac output with no significant change, or a small decrease, in left ventricular end-diastolic pressure (LVEDP). There is evidence that nicardipine increases blood flow.
-
Coronary dilatation induced by nicardipine I.V. improves perfusion and aerobic metabolism in areas with chronic ischemia, resulting in reduced lactate production and augmented oxygen consumption.
-
In patients with coronary artery disease, nicardipine I.V., administered after beta- blockade, significantly improved systolic and diastolic left ventricular function.
-
In congestive heart failure patients with impaired left ventricular function, nicardipine I.V. increased cardiac output both at rest and during exercise. Decreases in left ventricular end- diastolic pressure were also observed. However, in some patients with severe left ventricular dysfunction, it may have a negative inotropic effect and could lead to worsened failure.
-
"Coronary steal" has not been observed during treatment with nicardipine I.V. (Coronary steal is the detrimental redistribution of coronary blood flow in patients with coronary artery disease from underperfused areas toward better perfused areas.)
-
Nicardipine I.V. has been shown to improve systolic shortening in both normal and hypokinetic segments of myocardial muscle. Radionuclide angiography has confirmed that wall motion remained improved during increased oxygen demand. (Occasional patients have developed increased angina upon receiving oral nicardipine. Whether this represents coronary steal in these patients, or is the result of increased heart rate and decreased diastolic pressure, is not clear.)
-
In patients with coronary artery disease, nicardipine I.V. improves left ventricular diastolic distensibility during the early filling phase, probably due to a faster rate of myocardial relaxation in previously underperfused areas. There is little or no effect on normal myocardium, suggesting the improvement is mainly by indirect mechanisms such as afterload reduction and reduced ischemia.
-
Nicardipine I.V. has no negative effect on myocardial relaxation at therapeutic doses.
-
The clinical benefits of these properties have not yet been demonstrated.
-
Electrophysiologic Effects:
-
In general, no detrimental effects on the cardiac conduction system have been seen with nicardipine I.V. During acute electrophysiologic studies, it increased heart rate and prolonged the corrected QT interval to a minor degree. It did not affect sinus node recovery or SA conduction times.
-
Pulmonary Function:
-
In two well-controlled studies of patients with obstructive airway disease treated with oral nicardipine, no evidence of increased bronchospasm was seen. In one of the studies, oral nicardipine improved forced expiratory volume 1 second (FEV1) and forced vital capacity (FVC) in comparison with metoprolol. Adverse experiences reported in a limited number of patients with asthma, reactive airway disease, or obstructive airway disease are similar to all patients treated with oral nicardipine.
- Absorption:
- Oral bioavailability is ~ 100%
- Distribution:
- Nicardipine is highly protein bound (>95%) in human plasma.
- Following infusion, nicardipine plasma concentrations decline tri-exponentially, with a rapid early distribution phase (α-half-life of 2.7 minutes), an intermediate phase (β-half-life of 44.8 minutes), and a slow terminal phase (γ-half-life of 14.4 hours) that can only be detected after long-term infusions.
- The apparent volume of distribution (Vd) using a non-compartment model is 8.3 L/kg.
- Metabolism:
- Nicardipine I.V. has been shown to be rapidly and extensively metabolized by the liver.
- Nicardipine does not induce or inhibit its own metabolism and does not induce or inhibit hepatic microsomal enzymes.
- Elimination:
- After coadministration of a radioactive intravenous dose of nicardipine I.V. with an oral 30 mg dose given every 8 hours, 49% of the radioactivity was recovered in the urine and 43% in the feces within 96 hours. None of the dose was recovered as unchanged nicardipine.
- Nicardipine (Cardene Injection). Product Insert. EKR Therapeutics, Inc. Bedminster, NJ. 2010.
- Drug Monograph: Amlodipine (Norvasc)
- Drug Monograph: Felodipine (Plendil)
- Drug Monograph: Nimodipine (Nymalize)
- Drug Monograph: Amiodarone (Cordarone; Pacerone)
- Anatomy: Heart (External Anterior & Posterior) Anatomy
- EBM Focused Topic: Endovascular Treatment for Acute Ischemic Stroke
- EBM Focused Topic: Nitroglycerin for Hypertensive Acute Decompensated Heart Failure with Pulmonary Edema
Indications
Dosing
(Adult)
(Pediatrics)
Hepatic Dosing
Dosage Forms
Warnings
Overdose
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
References
Other EBM Consult Related Content
MESH Terms & Keywords
|
|---|
|

