Amiodarone (Cordarone, Pacerone): Drug Monograph
|
|---|
- Treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy.
- Use amiodarone for acute treatment until the patient's ventricular arrhythmias are stabilized. Most patients will require this therapy for 48 to 96 hours, but it may be safely administered for longer periods if necessary.
- Atrial Fibrillation:
- Oral: 600 - 800 mg in divided doses until a total of 10g given, then use 100 - 200 mg daily. Note: other dosing regimens similar to this have been used.
- IV: 150 mg per 100 mL (in 100 mL of D5W) infused over 10 minutes, then 1 mg/min for 6 hours, and then 0.5 mg/min (or) switch to oral dosing at 100 - 200 mg by mouth daily thereafter.
- Pulseless V-Tach or V-Fib:
- IV/IO: 300 mg as an undiluted rapid bolus, can repeat with a single 150 mg IV/IO push if continues. If return of spontaneous circulation occurs then give 1 mg/min IV/IO for 6 hours, then 0.5 mg/min IV/IO thereafter up to 1050 mg (max is 2.2 g per day).
- This trial is the reason amiodarone is included in the ACLS Guidelines for Pulseless V-Tach or V-Fib
- EBM Consult Article: Click Here
- Stable V-Tach:
- IV: 1050 mg over the first 24 hours of therapy, delivered by the following infusion regimen: Initial IV load of 150 mg per 100 mL (in 100 mL of D5W) infused over 10 minutes, then 1 mg/min for 6 hours, and then 0.5 mg/min thereafter.
- Supraventricular Tachycardia (SVT):
- IV: 150 mg per 100 mL (in 100 mL of D5W) infused over 10 minutes, then 1 mg/min for 6 hours, and then 0.5 mg/min (or) switch to oral dosing at 100 - 200 mg by mouth daily thereafter
- No specific recommendations from manufacturer but consider decreasing the dose or avoiding its use in severe liver impairment.
- Injection (generic): 50 mg/mL (vials: 3, 9, 18 mL)
- Injection (Nexterone): 1.5 mg/mL (IV bags: 100 or 200 mL)
- Oral (generic): 100, 200, 400 mg
- Oral
(Cordarone): 200 mg
- Oral (Pacerone): 100, 200, 400 mg
- Pulmonary toxicity (10% to 17%; consisting of hypersensitivity pneumonitis or interstitial or alveolar pneumonitis)
- Liver
injury (mild) but cases of fatal events present
- Worsening cardiac dysrhythmias (2% to 5% heart block or sinus bradycardia)
- Known hypersensitivity to any of the components of amiodarone, including iodine
- Cardiogenic shock
- Marked sinus bradycardia
- Second- or third-degree atrio-ventricular (AV) block unless a functioning pacemaker is available
- Hypotension - treat initially by slowing the infusion; additional standard therapy may be needed, including the following: vasopressor drugs, positive inotropic agents, and volume expansion.
- Bradycardia and AV block - treat by slowing the infusion rate or discontinuing amiodarone
- Liver enzyme elevations (usually mild)
- Proarrhythmia - may cause a worsening of existing arrhythmias or precipitate a new arrhythmia (2% to 5%)
- Pulmonary disorders (10% to 17%; consisting of hypersensitivity pneumonitis or interstitial or alveolar pneumonitis)
- Thyroid abnormalities - may cause increased T4 levels, decreased T3 levels, and increased levels of inactive reverse T3 in clinically euthyroid patients Surgery - close perioperative monitoring in patients undergoing general anesthesia - may be more sensitive to the myocardial depressant and conduction defects of halogenated inhalational anesthetics
- Hypotension
- Asystole/cardiac arrest/pulseless electrical activity: VT and cardiogenic shock
- Torsade de Pointes (TdP)
- Congestive heart failure
- Liver function test abnormalities (usually mild)
- Pulmonary toxicity (10% to 17%; consisting of hypersensitivity pneumonitis or interstitial or alveolar pneumonitis)
- Corneal deposits and optic neuritis leading to changes in vision (visual halos, photophobia, visual disturbances)
- Thyroid dysfunction (hypothyroidism and hyperthyrodism)
- The incidence has been reported to be 2 - 5.3%
- The mechanism is related to the high iodine concentration associated with each dose of amiodarone.
- EBM Consult Article: Click Here
- Effects include hypotension, cardiogenic shock, bradycardia, AV block, and hepatotoxicity.
- Treat hypotension ad cardiogenic shock by slowing the infusion rate or with standard therapy: vasopressor drugs, positive inotropic agents, and volume expansion.
- Bradycardia and AV block may require temporary pacing.
- Monitor
hepatic enzyme concentrations closely.
- Amiodarone is not dialyzable.
- CYP450 Enzymes:
- Substrate: CYP2C8 and CYP3A4. Drugs/substances that inhibit CYP3A and CYP2C8 - may decrease the metabolism and increase serum concentration of amiodarone
- Inhibitor: CYP1A2, CYP2C9, CYP2D6, and CYP3A4
- Transporter:
- Substrate: P-glycoprotein (P-gp)
- Inhibitor: P-glycoprotein (P-gp)
- QT-Prolongation:
- Fluoroquinolones, macrolide antibiotics, and azoles known to cause QTc prolongation - QTc prolongation, with or without TdP
- Amiodarone use in a patient on stable doses of digoxin can result in significant digoxin toxicity.
- This is clinically significant should not be missed.
- EBM Consult Article: Click Here
- Pregnancy: Pregnancy Category D
- Labor and Delivery: It is not known whether the use of amiodarone during labor or delivery has any immediate or delayed adverse effects.
- Nursing Mothers: Amiodarone and one of its major metabolites, desthylamiodarone (DEA) are excreted in human milk. Discontinuing breastfeeding is recommended.
- Renal Impairment: None
- Hepatic Impairment: No specific recommendations from manufacturer but consider decreasing the dose or avoiding its use in severe liver impairment
- Pediatric Patients: The safety and efficacy have not been established.
- Geriatric Patients: Carefully consider the dose selection in an elderly patient (consider lower doses and drug interaction potential with other concomitant medications). In general, start at the low end of the dosing range to reflect the greater frequency of decreased hepatic, renal or cardiac function, and concomitant disease or other drug therapy.
- It is not known whether the use of amiodarone during labor or delivery has any immediate or delayed adverse effects.
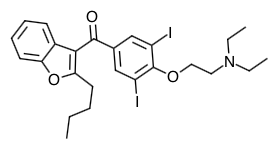
- Scientific Name: (2-butyl-3- benzo-furanyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone hydrochloride
- Empirical Formula: C25H29I2NO3.HCl
- Molecular Weight: 681.78
- Amiodarone is generally considered a class III antiarrhythmic drug especially with prolonged use and results in lengthening of the action potential. It also has activity similar to class I agents where amiodarone blocks sodium channels at rapid pacing frequencies, to class II agents where amiodarone exerts a noncompetitive antisympathetic action (by block of calcium and potassium channels are responsible for the negative dromotropic effects on the sinus node and for the slowing of conduction and prolongation of refractoriness in the atrioventricular (AV) node), and class IV agents where it can exert negative chronotopic effects in nodal cells.
- In animals and humans, the use of IV amiodarone has been shown to produce negative inotropic and vasodilatory effects. In clinical studies of patients with refractory VF or hemodynamically unstable VT, treatment-emergent, drug-related hypotension occurred in 288 of 1836 patients (16%) treated with intravenous amiodarone. No correlations were seen between the baseline ejection fraction and the occurrence of clinically significant hypotension during infusion of intravenous amiodarone.
- The development of maximal ventricular class III effects after oral amiodarone administration in humans correlates more closely with desethylamiodarone (DEA) accumulation over time than with amiodarone accumulation. On the other hand, after intravenous amiodarone administration, there is evidence of activity well before significant concentrations of DEA are attained.
- Absorption: Amiodarone exhibits complex disposition characteristics after intravenous administration. Peak serum concentrations after single 5 mg/kg 15-minute intravenous infusions in healthy subjects range between 5 and 41 mg/L. Peak concentrations after 10-minute infusions of 150 mg intravenous amiodarone in patients with ventricular fibrillation (VF) or hemodynamically unstable ventricular tachycardia (VT) range between 7 and 26 mg/L. Due to rapid distribution, serum concentrations decline to 10% of peak values within 30 to 45 minutes after the end of the infusion. In clinical trials, after 48 hours of continued infusions (125, 500 or 1000 mg/day) plus supplemental (150 mg) infusions (for recurrent arrhythmias), amiodarone mean serum concentrations between 0.7 to 1.4 mg/L were observed (n=260).
- Distribution: From in vitro studies, the protein binding
of amiodarone is >96%. Amiodarone and DEA cross the placenta and both appear
in breast milk. Neither amiodarone nor DEA is dialyzable.
- Metabolism: N-desethylamiodarone (DEA) is the major active metabolite of amiodarone in humans. DEA serum concentrations above 0.05 mg/L are not usually seen until after several days of continuous infusion but with prolonged therapy reach approximately the same concentration as amiodarone. Amiodarone is metabolized to DEA by the cytochrome P450 enzyme group, specifically cytochrome CYP3A and CYP2C8. The CYP3A isoenzyme is present in both the liver and intestines. The highly variable systemic availability of oral amiodarone may be attributed to large interindividual variability in CYP3A activity.
- Elimination: Amiodarone is eliminated primarily by hepatic metabolism and biliary excretion and there is negligible excretion of amiodarone or DEA in urine. In studies in healthy subjects following single intravenous administration (5 mg/kg of amiodarone over 15 min), the plasma concentration vs. time profile could be characterized by linear sum of four exponential terms with terminal elimination half-lives (t1/2) of 9 - 36 days for amiodarone and 9 - 30 days for DEA. The clearance of amiodarone and DEA ranged between 63 - 231 mL/hr/kg and 140 - 400 mL/hr/kg, respectively. In clinical studies of 2 to 7 days, clearance of amiodarone after intravenous administration in patients with VT and VF ranged between 220 and 440 mL/hr/kg.
- Advise patients that amiodarone has the potential to cause serious side effects that limit its use to life-threatening and hemodynamically unstable cardiac arrhythmias.
- Advise female patients to discontinue nursing while being treated with amiodarone, as breastfeeding could expose the nursing infant to a significant dose of the drug.
- Recommend that patients avoid grapefruit juice, over-the-counter cough medicine (which commonly contain dextromethorphan), and St. John's Wort.
- Inform patients that most manufacturers of corneal refractive laser surgery devices contraindicate corneal refractive laser surgery in patients taking amiodarone.
- Discuss the symptoms of hypo- and hyperthyroidism with patients, particularly if patients will be transitioned to oral amiodarone.
- Amiodarone
(Cordarone, Pacerone). Product
Insert. SAGENT Pharmaceuticals,
Inc. Schaumburg, IL. 2011.
- Field
JM et al. Part 1: Executive Summary: 2010 American Heart Association Guidelines
for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 suppl 3):640-56. PMID: 20956217
- January CT et al. 2014 AHA/ACC/HRS guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014;130(23):e199-267. PMID: 24682347
Indications
Dosing
Core Clinical Trial - The ARREST Trial
Hepatic Dosing
Dosage Forms
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
The Evidence & Mechanism: Amiodarone-Induced Hyperthyroidism
Overdose
Drug Interactions
The Evidence & Mechanism: Amiodarone - Digoxin Drug Interaction
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
References
Other EBM Consult Related Content
|
|---|
|
MESH Terms & Keywords
|
|---|
|