Ziprasidone (Geodon): Drug Monograph
|
|---|
- Treatment of schizophrenia (oral)
- As monotherapy for acute treatment of manic or mixed episodes associated with bipolar I disorder (oral)
- Maintenance treatment of bipolar I disorder as an adjunct to lithium or valproate (oral)
- Acute treatment of agitation in schizophrenic patients for whom treatment with ziprasidone is appropriate and who need IM antipsychotic medication for rapid control of agitation (Injection)
- Acute agitation in schizophrenia:
- IM: 10 mg (may give every 2 hours) to 20 mg (may give every 4 hours)
- Maximum: 40 mg/day
- If long-term therapy is indicated, replace with oral formulation as soon as possible
- Schizophrenia:
- Capsules: 20 mg twice daily
- May adjust up to 80 mg twice daily at intervals of not <2 days
- Maximum: 80 mg twice daily
- Bipolar I disorder:
- Capsules, monotherapy: 40 mg twice daily
- May increase to 60 mg or 80 mg twice daily on day 2 of treatment, and subsequently adjust based on tolerability and efficacy within the range of 40-80 mg twice daily
- Maintenance, adjunct to lithium or valproate: Continue treatment at the same dose on which the patient was initially stabilized, within the range of 40-80 mg twice daily
- Intramuscular ziprasidone should be administered with caution to patients with impaired function.
- Elderly patients with dementia-related psychosis are at an increased risk of death. Although the causes of death are varied, most of the deaths are either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature.
- Ziprasidone is not approved for the treatment of patients with Dementia-Related Psychosis.
- Known history of QT prolongation
- Patients with recent acute myocardial infarction
- Patients with uncompensated heart failure
- In combination with other drugs that have demonstrated QT prolongation
- Known hypersensitivity to ziprasidone
- Increased mortality in elderly patients with dementia-related psychosis
- QT interval prolongation - use should be avoided in patients with bradycardia, hypokalemia or hypomagnesemia, congenital prolongation of the QT interval, or in combination with other drugs that have demonstrated QT prolongation
- Neuroleptic malignant syndrome (NMS) - potentially fatal symptom complex has been reported with antipsychotic drugs. Manage with immediate discontinuation of drug and close monitoring.
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) - possible with ziprasidone exposure. May be fatal. Discontinue treatment if DRESS is suspected.
- Tardive dyskinesia - may develop acutely or chronically
- Metabolic changes - atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. Thee changes include hyperglycemia, dyslipidemia, and weight gain.
- Hyperglycemia and diabetes mellitus (DM) - monitor all patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients with DM risk factors should undergo blood glucose testing before and during treatment.
- Dyslipidemia - undesirable alterations may occur
- Weight gain - monitor weight gain
- Rash - discontinue in patients who develop a rash without an identified cause
- Orthostatic hypotension - use with caution in patients with known cardiovascular of cerebrovascular disease
- Leukopenia, neutropenia, and agranulocytosis - patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue treatment at the first sign of a decline in WBC in the absence of other causative factors.
- Seizures - use cautiously in patients with a history of seizures or with conditions that lower seizure threshold
- Potential for cognitive and motor impairment - patients should use caution when operating machinery
- Suicide - closely supervise high-risk patients
- Dysphagia - esophageal dysmotility and aspiration possible. Use with caution in patients at risk for aspiration pneumonia.
- Schizophrenia:
- Somnolence
- Respiratory tract infection
- Manic and mixed episodes associated with bipolar disorder:
- Somnolence
- Extrapyramidal symptoms
- Dizziness
- Akathisia
- Abnormal vision
- Asthenia
- Vomiting
- Intramuscular administration:
- Headache
- Nausea
- Somnolence
-
Symptoms may include minimal sedation, slurring of speech, transitory hypertension, extrapyramidal symptoms, somnolence, tremor, and anxiety.
-
Establish and maintain an airway and ensure adequate oxygenation and ventilation.
-
Intravenous access should be established, and gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered.
-
The possibility of obtundation, seizure, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
-
Cardiovascular monitoring should commence immediately.
-
Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids. If sympathomimetic agents are used for vascular support, epinephrine and dopamine should not be used, since beta stimulation combined with ?1 antagonism associated with ziprasidone may worsen hypotension.
-
In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered.
-
Ziprasidone is not dialyzable.
-
The possibility of multiple drug involvement should be considered.
- Drugs that have demonstrated QT prolongation
- Other centrally acting drugs - use with caution due to the primary CNS effects of ziprasidone
- Certain antihypertensive agents - ziprasidone may enhance the effects of these drugs
- Levodopa and dopamine agonists - ziprasidone may antagonize the effects of these
- Carbamazepine - may decrease the AUC of ziprasidone. This effect may be greater when higher doses of carbamazepine are administered.
- Ketoconazole - may result in an increase of the AUC and Cmax of ziprasidone by about 35-40%.
- Food - the absorption of ziprasidone is increased up to two-fold in the presence of food
- Pregnancy: Pregnancy Category C
- Labor and Delivery: Effect is unknown.
- Nursing Mothers: Breast-feeding is not recommended.
- Renal Impairment: Intramuscular ziprasidone should be administered with caution to patients with impaired renal function as the cyclodextrin excipient is cleared by renal filtration.
- Hepatic Impairment: The presence of hepatic impairment would be expected to increase the AUC of ziprasidone as it is cleared substantially by the liver.
- Pediatric Patients: Safety and effectiveness have not been established.
- Geriatric Patients: No overall differences in safety or effectiveness have been observed between elderly and younger subjects but greater sensitivity of some older individuals cannot be ruled out. The presence of multiple factors that might increase the pharmacodynamic response to ziprasidone, or cause poorer tolerance or orthostasis should lead to consideration of a lower starting dose, slower titration, and careful monitoring during the initial dosing period for some elderly patients. Ziprasidone intramuscular has not been systemically evaluated in elderly patients (65 years and over).
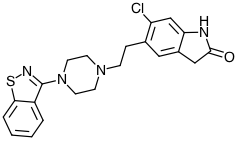
- Scientific Name:
- 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one Ziprasidone capsules contain a monohydrochloride, monohydrate salt of ziprasidone: ziprasidone hydrochloride monohydrate is 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one, monohydrochloride, monohydrate
- Ziprasidone for injection contains a lyophilized form of ziprasidone mesylate trihydrate: 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one, methanesulfonate, trihydrate
- Empirical Formula:
- Free base of ziprasidone: C21H21CIN4OS
- Ziprasidone hydrochloride monohydrate: C21H21CIN4OS.HCl.H2O
- Ziprasidone for injection: C21H21ClN4OS.CH3SO3H.3H2O
- Molecular Weight:
- Free base: 412.94
- Ziprasidone hydrochloride monohydrate: 467.42
- Ziprasidone for injection: 563.09
- The mechanism of action of ziprasidone, as with other drugs having efficacy in schizophrenia, is unknown. However, it has been proposed that this drug's efficacy in schizophrenia is mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism. As with other drugs having efficacy in bipolar disorder, the mechanism of action of ziprasidone in bipolar disorder is unknown.
- Ziprasidone exhibited high in vitro binding affinity for the dopamine D2 and D3, the serotonin 5HT2A, 5HT2C, 5HT1A, 5HT1D, and ?1-adrenergic receptors (Ki s of 4.8, 7.2, 0.4, 1.3, 3.4, 2, and 10 nM, respectively), and moderate affinity for the histamine H1 receptor (Ki=47 nM). Ziprasidone functioned as an antagonist at the D2, 5HT2A, and 5HT1D receptors, and as an agonist at the 5HT1A receptor. Ziprasidone inhibited synaptic reuptake of serotonin and norepinephrine. No appreciable affinity was exhibited for other receptor/binding sites tested, including the cholinergic muscarinic receptor (IC50 >1 µM). Antagonism at receptors other than dopamine and 5HT2 with similar receptor affinities may explain some of the other therapeutic and side effects of ziprasidone. Ziprasidone's antagonism of histamine H1 receptors may explain the somnolence observed with this drug. Ziprasidone's antagonism of ?1-adrenergic receptors may explain the orthostatic hypotension observed with this drug.
-
Oral Pharmacokinetics: Ziprasidone's activity is primarily due to the parent drug. The multiple-dose pharmacokinetics of ziprasidone are dose-proportional within the proposed clinical dose range, and ziprasidone accumulation is predictable with multiple dosing. Elimination of ziprasidone is mainly via hepatic metabolism with a mean terminal half-life of about 7 hours within the proposed clinical dose range. Steady-state concentrations are achieved within one to three days of dosing. The mean apparent systemic clearance is 7.5 mL/min/kg. Ziprasidone is unlikely to interfere with the metabolism of drugs metabolized by cytochrome P450 enzymes.
-
Absorption: Ziprasidone is well absorbed after oral administration, reaching peak plasma concentrations in 6 to 8 hours. The absolute bioavailability of a 20 mg dose under fed conditions is approximately 60%. The absorption of ziprasidone is increased up to two-fold in the presence of food.
-
Distribution: Ziprasidone has a mean apparent volume of distribution of 1.5 L/kg. It is greater than 99% bound to plasma proteins, binding primarily to albumin and ?1-acid glycoprotein. The in vitro plasma protein binding of ziprasidone was not altered by warfarin or propranolol, two highly protein-bound drugs, nor did ziprasidone alter the binding of these drugs in human plasma. Thus, the potential for drug interactions with ziprasidone due to displacement is minimal.
-
Metabolism: Ziprasidone is extensively metabolized after oral administration with only a small amount excreted in the urine (<1%) or feces (<4%) as unchanged drug. Ziprasidone is primarily cleared via three metabolic routes to yield four major circulating metabolites, benzisothiazole (BITP) sulphoxide, BITP-sulphone, ziprasidone sulphoxide, and S-methyldihydroziprasidone.
-
Elimination: Approximately 20% of the dose is excreted in the urine, with approximately 66% being eliminated in the feces. Unchanged ziprasidone represents about 44% of total drug-related material in serum. In vitro studies using human liver subcellular fractions indicate that S-methyldihydroziprasidone is generated in two steps. These studies indicate that the reduction reaction is mediated primarily by chemical reduction by glutathione as well as by enzymatic reduction by aldehyde oxidase and the subsequent methylation is mediated by thiol methyltransferase. In vitro studies using human liver microsomes and recombinant enzymes indicate that CYP3A4 is the major CYP contributing to the oxidative metabolism of ziprasidone. CYP1A2 may contribute to a much lesser extent. Based on in vivo abundance of excretory metabolites, less than one-third of ziprasidone metabolic clearance is mediated by cytochrome P450 catalyzed oxidation and approximately two-thirds via reduction. There are no known clinically relevant inhibitors or inducers of aldehyde oxidase.
-
Intramuscular Pharmacokinetics
-
Systemic Bioavailability: The bioavailability of ziprasidone administered intramuscularly is 100%. After intramuscular administration of single doses, peak serum concentrations typically occur at approximately 60 minutes post-dose or earlier and the mean half-life (T?) ranges from two to five hours. Exposure increases in a dose-related manner and following three days of intramuscular dosing, little accumulation is observed.
-
Metabolism and Elimination: Although the metabolism and elimination of IM ziprasidone have not been systematically evaluated, the intramuscular route of administration would not be expected to alter the metabolic pathways.
-
Patients should be instructed to take ziprasidone capsules with food for optimal absorption.
-
Patients should be advised to inform their healthcare providers of the following: history of QT prolongation; recent acute myocardial infarction; uncompensated heart failure; prescription of other drugs that have demonstrated QT prolongation; risk for significant electrolyte abnormalities; and history of cardiac arrhythmia.
-
Patients should be instructed to report the onset of any conditions that put them at risk for significant electrolyte disturbances, hypokalemia in particular, including but not limited to he initiation of diuretic therapy or prolonged diarrhea.
-
Patients should be instructed to report symptoms such as dizziness, palpitations, or syncope to the prescriber.
-
Patients should be instructed to report to their healthcare provider at the earliest onset any signs or symptoms that may be associated with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
-
Patients should be advised to inform their healthcare provider of any medicines they are taking, including non-prescription medicines, supplements, and herbal medicines.
-
Patients should be advised to inform their healthcare provider if they are pregnant, might be pregnant, or plan to get pregnant.
-
Patients should also be advised to inform their healthcare provider if they are breast-feeding or plan to breast-feed.
-
Patients should be advised to be careful when operating machinery or driving a motor vehicle as ziprasidone may cause sleepiness.
Indications
Dosing (Adult)
General Notes: Capsules: Take with food. IM: Add 1.2 mL of sterile water for injection (SWFI) to vial and shake vigorously until all the drug is dissolved. Draw up 0.5 mL of reconstituted solution to administer a 10 mg dose; draw up 1 mL of reconstituted solution to administer a 20 mg dose. Do not mix with other medicinal products or solvents other than SWFI
Renal Dosing
Black Box Warnings
Contraindications
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|