This
is one of many drug-drug interactions where clinicians cannot rely solely on
knowing the cytochrome P450 enzyme system for predicting drug interactions.
As it relates to this question, it is important to note that the
coadministration of valproic acid and lamotrigine is possible given their FDA
approved indications. Regardless of the formulation used (divalproex
sodium (as 1:1 sodium valproate:valproic acid; Depakote) or valproic acid only
(Depakene)), the active form in the body is valproate and is approved as
monotherapy and adjunctive therapy in complex partial seizures and pediatric
patients (>10 years of age). Similarly, lamotrigine is indicated as
adjunctive therapy for partial seizures, generalized seizures of Lennox-Gastaut
syndrome, and primary generalized tonic-clonic seizures in adult and pediatric
patients (>2 years of age). It is also important to note that both are
FDA approved for treatment of Bipolar I Disorder, thus highlighting the
possibility of their coadministration.
Unfortunately,
lamotrigine has a black box warning (BBW) because of its association with
causing serious skin rashes, including Stevens-Johnson Syndrome. While
the overall occurrence is low, it does appear that pediatric patients are at
greater risk with a reported incidence of 0.8% (8 per 1,000) for pediatric
patients (< 16 years of age) and 0.3% (3 per 1,000) in adults on
adjunct therapy for epilepsy. In addition to age, other associated risk
factors include coadministration of lamotrigine with valproate (regardless of
formulation), exceeding the recommended initial doses of lamotrigine, and
titrating the dose of lamotrigine too quickly. Interestingly, neither
valproate nor lamotrigine are known substrates or inhibitors of the cytochrome
P450 enzyme system commonly associated with drug-drug interactions. Also,
it does not appear that the drug interaction is occurring at the level of
transporters since valproic acid is not a substrate or inhibitor of
P-glycoprotein (P-gp; an efflux pump) like lamotrigine, nor are either of them
known substrates or inhibitors of other efflux pumps. (i.e. multidrug
resistance-associated protein 2 (MRP2) or breast cancer resistance protein
(BCRP)). How then does valproic acid cause an increase in the levels of
lamotrigine and subsequently increase the risk for serious skin rashes in some
patients.
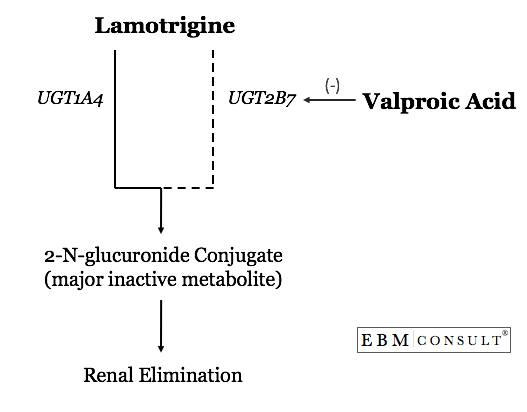
The
interaction occurs through a phase II glucuronidation metabolic pathway
catalyzed by a series of enzymes known as uridine diphosphate
glucuronosyltransferase (UGT). This process makes the lamotrigine more
water soluble to facilitate elimination from the body. Lamotrigine is
primarily metabolized by UGT1A4 and 2B7 to its major inactive metabolite,
2-N-glucuronide conjugate, with most being found in the urine. Because of
this level of dependency on glucuronidation for lamotrigine's inactivation and
elimination, anything that inhibits either one of these UGT enzymes will affect
lamotrigine levels. Valproate is a known inhibitor of UGT2B7 but does not
effect UGT1A4. One study showed that the AUC for lamotrigine increased
2.6 fold in patients taking valproate 500 mg twice daily.7 Additionally,
the half-life of lamotrigine increases from 25 hrs to 70 hrs with
coadministration of valproate. It appears that valproate doses between
250-500 mg/day results in the maximal inhibition of lamotrigine metabolism.2
While
the incidence appears to be low, the higher incidence in pediatric patients and
those being exposed to higher doses of lamotrigine warrants consideration of
this interaction since the skin rashes can be life threatening. As such,
it is recommended that patients being newly started on lamotrigine undergo a
minimum of a 5 week dose titration phase where patients are started out at
lower than normal maintenance doses and increased slowly in 2 week intervals.
References:
- Abbott Pharmaceuticals PR Ltd. Divalproex sodium (Depakote) product
insert. Barceloneta, PR. October 2006. Last accessed on 12/13/2008.
- GlaxoSmithKline. Lamotrigine (Lamictal) product insert. Greenville, NC. May 2007. Last accessed on 12/13/2008.
- Potschka
H, Fedrowitz M, Loscher W. P-glycoprotein-mediated efflux of
phenobarbital, lamotrigine, and felbamate at the blood brain barrier:
evidence from microdialysis experiments in rats. Neurosci Lett. 2002;327:173-176.
- Baltes
S, Fedrowitz M, Tortos CL et al. Valproic acid is not a substrate for
P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of
in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320(1):331-43.
- Cerveny
L, Pavek P, Malakova J et al. Lack of interactions between breast
cancer resistance protein (BCRP/ABCG2) and selected antiepileptic
agents. Epilepsia. 2006;47(3):461-468.
- Rowland
A, Elliot DJ, Williams JA et al. In vitro characterization of
lamotrigine N2-glucuronidation and the lamotrigine-valproic acid
interaction. Drug Metab Dispos. 2006;34(6):1055-62.
- Morris
RG, Black AB, Lam E et al. Clinical study of lamotrigine and valproic
acid in patients with epilepsy: using a drug interaction to advantage? Ther Drug Monit. 2000;22:656-660.
- Patterson RM, Hoyle PC, Editorial Staff of the Publishers of Lawyers' Medical Cyclopedia eds. Drugs in Litigation: Damage Awards Involving Prescription and Nonprescription Drugs. 2007 Edition. LexisNexis. San Francisco, CA.
- Saukas v. Walker St. Pharm., 2005 Mich. App. LEXIS 1847 (Mich. Ct. App. 2005).


