Rosiglitazone (Avandia): Drug Monograph
|
|---|
- Improve glycemic control in adults with type 2 diabetes mellitus as an adjunct to diet and exercise
- Rosiglitazone should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis
- Co-administration of rosiglitazone and insulin is not recommended
- General Notes:
- Doses increases should be accompanied by careful monitoring for adverse events related to fluid retention.
- Take with or without food
- Diabetes Mellitus, Type 2
- 4 mg by mouth daily in single or divided doses
- May increase to 8 mg/day if response is inadequate after 8-12 weeks
- Max Dose: 8 mg by mouth once a day
- Patients with clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X ULN at start of therapy):
- Do not initiate therapy
- May cause or exacerbate congestive heart failure in some patients. After initiation of rosiglitazone, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of rosiglitazone must be considered.
- Rosiglitazone is not recommended in patients with symptomatic heart failure. Initiation of rosiglitazone in patients with established NYHA Class III or IV heart failure is contraindicated.
- There is a potential increased risk of myocardial infarction with the use of rosiglitazone.
- Fluid retention, which may exacerbate or lead to heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk of other cardiovascular effects.
- Increased risk of myocardial infarction
- Co-administration with insulin is not recommended
- Dose-related edema, weight gain, and anemia
- Macular edema
- Increased incidence of bone fracture
- Limited data are available with regard to overdosage in humans.
- In event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient's clinical status.
- Inhibitors of CYP2C8 (e.g., gemfibrozil) may increase rosiglitazone levels
- Inducers of CYP2C8 (e.g., rifampin) may decrease rosiglitazone levels.
- Pregnancy: Pregnancy Category C.
- Labor and Delivery: The effect of rosiglitazone is not known.
- Nursing Mothers: It is not known whether rosiglitazone is excreted in human milk.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Data are insufficient to recommend pediatric use of rosiglitazone.
- Geriatric Patients: No overall differences in safety and effectiveness between older (≥65 years) and younger (≤65 years) patients were observed.
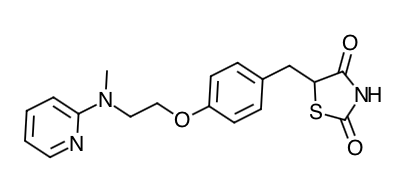
- Scientific Name: (±)-5-[[4-[2-(methyl-2- pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1)
- Empirical Formula: C18H19N3O3S.C4H4O4
- Molecular Weight: 473.52 (357.44 free base)
- Rosiglitazone, a member of the thiazolidinedione class of anti-diabetic agents, improves glycemic control by improving insulin sensitivity. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator-activated receptor-gamma (PPARγ). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism.
- Insulin resistance is a common feature characterizing the pathogenesis of type 2 diabetes. The anti-diabetic activity of rosiglitazone has been demonstrated in animal models of type 2 diabetes in which hyperglycemia and/or impaired glucose tolerance is a consequence of insulin resistance in target tissues. Rosiglitazone reduces blood glucose concentrations and reduces hyperinsulinemia in the ob/ob obese mouse, db/db diabetic mouse, and fa/fa fatty Zucker rat.
- In animal models, the anti-diabetic activity of rosiglitazone was shown to be mediated by increased sensitivity to insulin's action in the liver, muscle, and adipose tissues. Pharmacological studies in animal models indicate that rosiglitazone inhibits hepatic gluconeogenesis. The expression of the insulin-regulated glucose transporter GLUT-4 was increased in adipose tissue. Rosiglitazone did not induce hypoglycemia in animal models of type 2 diabetes and/or impaired glucose tolerance.
- Patients with lipid abnormalities were not excluded from clinical trials of rosiglitazone. In all 26-week controlled trials, across the recommended dose range, rosiglitazone as monotherapy was associated with increases in total cholesterol, LDL, and HDL and decreases in free fatty acids. These changes were statistically significantly different from placebo or glyburide controls.
- Increases in LDL occurred primarily during the first 1 to 2 months of therapy with rosiglitazone and LDL levels remained elevated above baseline throughout the trials. In contrast, HDL continued to rise over time. As a result, the LDL/HDL ratio peaked after 2 months of therapy and then appeared to decrease over time. Because of the temporal nature of lipid changes, the 52-week glyburide-controlled trial is most pertinent to assess long-term effects on lipids. At baseline, week 26, and week 52, mean LDL/HDL ratios were 3.1, 3.2, and 3.0, respectively, for rosiglitazone 4 mg twice daily. The corresponding values for glyburide were 3.2, 3.1, and 2.9. The differences in change from baseline between rosiglitazone and glyburide at week 52 were statistically significant.
- The pattern of LDL and HDL changes following therapy with rosiglitazone in combination with other hypoglycemic agents were generally similar to those seen with rosiglitazone in monotherapy.
- The changes in triglycerides during therapy with rosiglitazone were variable and were generally not statistically different from placebo or glyburide controls.
- Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range. The elimination half-life is 3 to 4 hours and in independent of dose.
- Absorption: The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Administration of rosiglitazone with food resulted in no change in overall exposure (AUC), but there was an approximately 28% decrease in Cmax and a delay in Tmax (1.75 hours). These changes are not likely to be clinically significant; therefore, rosiglitazone may be administered with or without food.
- Distribution: The mean (CV%) oral volume of distribution (Vss/F) of rosiglitazone is approximately 17.6 (30%) liters, based on a population pharmacokinetic analysis. Rosiglitazone is approximately 99.8% bound to plasma proteins, primarily albumin.
- Metabolism: Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine. The major routes of metabolism were N-demethylation and hydroxylation, followed by conjugation with sulfate and glucuronic acid. All the circulating metabolites are considerably less potent than parent and, therefore, are not expected to contribute to the insulin-sensitizing activity of rosiglitazone. In vitro data demonstrate that rosiglitazone is predominantly metabolized by Cytochrome P450 (CYP) isoenzyme 2C8, with CYP2C9 contributing as a minor pathway.
- Elimination: Following oral or intravenous administration of [14C] rosiglitazone maleate, approximately 64% and 23% of the dose was eliminated in the urine and in the feces, respectively. The plasma half-life of [14C] related material ranged from 103 to 158 hours.
- Population Pharmacokinetics in Patients with Type 2 Diabetes: Population pharmacokinetic analyses from 3 large clinical trials including 642 men and 405 women with type 2 diabetes (aged 35 to 80 years) showed that the pharmacokinetics of rosiglitazone are not influenced by age, race, smoking, or alcohol consumption. Both oral clearance (CL/F) and oral steady-state volume of distribution (Vss/F) were shown to increase with increases in body weight. Over the weight range observed in these analyses (50 to 150 kg), the range of predicted CL/F and Vss/F values varied by <1.7-fold and <2.3-fold, respectively. Additionally, rosiglitazone CL/F was shown to be influenced by both weight and gender, being lower (about 15%) in female patients.
- Special Populations
- Geriatric: Results of the population pharmacokinetic analysis (n=716 <65 years; n=331≥ 65 years) showed that age do not significantly affect the pharmacokinetics of rosiglitazone.
- Gender: Results of the population pharmacokinetics analysis showed that the mean oral clearance of rosiglitazone in female patients (n = 405) was approximately 6% lower compared to male patients of the same body weight (n = 642). As monotherapy and in combination with metformin, rosiglitazone improved glycemic control in both males and females. In metformin combination trials, efficacy was demonstrated with no gender differences in glycemic response. In monotherapy trials, a greater therapeutic response was observed in females; however, in more obese patients, gender differences were less evident. For a given body mass index (BMI), females tend to have a greater fat mass than males. Since the molecular target PPARγ is expressed in adipose tissues, this differentiating characteristic may account, at least in part, for the greater response to rosiglitazone in females. Since therapy should be individualized, no dose adjustments are necessary based on gender alone.
- Hepatic Impairment: Unbound oral clearance of rosiglitazone was significantly lower in patients with moderate to severe liver disease (Child-Pugh Class B/C) compared to healthy subjects. As a result, unbound Cmax and AUC0-inf were increased 2- and 3-fold, respectively. Elimination half-life for rosiglitazone was about 2 hours longer in patients with liver disease, compared to healthy subjects. Therapy with rosiglitazone should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT>2.5X upper limit of normal) at baseline.
- Pediatric: Pharmacokinetic parameters of rosiglitazone in pediatric patients were established using a population pharmacokinetic analysis with sparse data from 96 pediatric patients in a single pediatric clinical trial including 33 males and 63 females with ages ranging from 10 to 17 years (weights ranging from 35 to 178.3 kg). Population mean CL/F and V/F of rosiglitazone were 3.15 L/hr and 13.5 L, respectively. These estimates of CL/F and V/F were consistent with the typical parameter estimates from a prior adult population analysis.
- Renal Impairment: There are no clinically relevant differences in the pharmacokinetics of rosiglitazone in patients with mild to severe renal impairment or in hemodialysis-dependent patients compared to subjects with normal renal function. No dosage adjustment is therefore required in such patients receiving rosiglitazone. Since metformin is contraindicated in patients with renal impairment, co-administration of metformin with rosiglitazone is contraindicated in these patients.
- Race: Results of a population pharmacokinetic analysis including subjects of Caucasian, black, and other ethnic origins indicate that race has no influence on the pharmacokinetics of rosiglitazone.
- Patients should be informed of the risks and benefits of rosiglitazone.
- Rosiglitazone is not recommended for patients with symptomatic heart failure. Treatment with rosiglitazone is associated with an increased risk for myocardial infarction (heart attack), especially in patients taking insulin. Rosiglitazone is not recommended for patients who are taking insulin.
- Management of type 2 diabetes should include diet control. Caloric restriction, weight loss, and exercise are essential for the proper treatment of the diabetic patient because they help improve insulin sensitivity. This is important not only in the primary treatment of type 2 diabetes, but in maintaining the efficacy of drug therapy. It is important to adhere to dietary instructions and to regularly have blood glucose and glycosylated hemoglobin tested. It can take 2 weeks to see a reduction in blood glucose and 2 to 3 months to see the full effect of rosiglitazone.
- Blood will be drawn to check their liver function prior to the start of therapy and periodically thereafter per the clinical judgment of the healthcare professional. Patients with unexplained symptoms of nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine should immediately report these symptoms to their physician.
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on rosiglitazone should immediately report these symptoms to their physician.
- When using rosiglitazone in combination with other hypoglycemic agents, the risk of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and their family members.
- Therapy with rosiglitazone like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking rosiglitazone. Thus, adequate contraception in premenopausal women should be recommended.
Indications
Dosing (Adult)
Hepatic Dosing
Black Box Warnings
Warnings
Overdose
Drug Interactions
Special Populations
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

