Rivaroxaban (Xarelto): Drug Monograph
|
|---|
- Reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treatment of deep vein thrombosis (DVT)and pulmonary embolism (PE)
- Reduction in the risk of recurrence of DVT and PE following initial 6 months treatment for DVT and/or PE
- Prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery
- General Notes:
- 15 mg and 20 mg tablets should be taken with food whereas 10 mg tablets can be taken with or without food.
- If unable to swallow whole tablets, they may be crushed and mixed with applesauce immediately prior to use. After administering crushed 15 mg or 20 mg tablets, immediately follow dose with food.
- Tablets may be crushed and suspended in 50 mL of water after confirming gastric placement of tube. Avoid administration distal to the stomach. After administration of 15 mg or 20 mg tablets, immediately follow dose with enteral feeding.
- Crushed tablets are stable in water and applesauce for up to 4 hours
- Reduction of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation:
- 20 mg daily with evening meal
- Prophylaxis of DVT Following Surgery:
- 10 mg daily. Give initial dose 6-10 hours after surgery provided that hemostasis has been established
- Treatment duration, hip replacement surgery: 35 days
- Treatment duration, knee replacement surgery: 12 days
- Treatment of DVT and PE:
- 15 mg twice daily with food for the first 21 days; then 20 mg daily with food, at approximately the same time each day
- Reduction in risk of recurrence following initial 6 months of treatment: 20 mg daily with food at approximately the same time each day
- Dose Conversions:
- Switching from warfarin:
- Discontinue warfarin and stat therapy as soon as INR is <3.0
- Switching to warfarin:
- No clinical trial data available to guide conversion; discontinue rivaroxaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of rivaroxaban would have been taken
- Switching from other anticoagulants:
- Start rivaroxaban 0-2 hours prior to next scheduled pm dose of drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of other anticoagulant. For continuous IV infusion of unfractionated heparin, stop infusion and start rivaroxaban at the same time
- Switching to other anticoagulants:
- If switching to rapid onset anticoagulant, discontinue rivaroxaban and give 1st dose of other anticoagulant (oral or parenteral) at the time that the next dose would have been taken
- Missed dose:
- Receiving 15 mg twice daily: Take rivaroxaban immediately to ensure intake of 30 mg/day; may take two 15 mg tablets at once. Continue with regular 15 mg twice daily as recommended on following day
- Receiving 20 mg, 15 mg, or 10 mg daily: Take missed dose immediately
- None
- Reduction in Risk of Stroke in Nonvalvular A-Fib, CrCl 15-50 mL/min:
- 15 mg daily with evening meal
- Premature discontinuation increases the risk of thrombotic events and spinal/epidural hematoma
- Epidural or spinal hematomas possible in patients who are receiving neuraxial anesthesia or undergoing spinal puncture; long-term or permanent paralysis may result
- Monitor frequently for signs/symptoms of neurologic impairment
- Risk of bleeding
- Pregnancy related hemorrhage and/or emergent delivery
- Prosthetic heart valves; rivaroxaban use not recommended
- Bleeding
- Increased risk of stroke after discontinuation in nonvalvular atrial fibrillation
- Spinal/epidural hematoma
- Overdosage may lead to hemorrhage.
- Discontinue rivaroxaban and initiate appropriate therapy if bleeding complications associated with overdosage occur.
- Due to the high plasma protein binding, rivaroxaban is not expected to be dialyzable.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: Safety and effectiveness of
rivaroxaban during labor and delivery have not been studied in clinical trials
- Nursing Mothers: It is unknown if rivaroxaban is excreted in human milk. Discontinue drug or discontinue nursing
- Renal Impairment: Avoid or adjust dose based on CrCl
- Hepatic Impairment: Avoid use in patients with Child-Pugh B and C hepatic impairment or with any degree of hepatic disease associated with coagulopathy
- Pediatric Patients: Safety and effectiveness in pediatric patients have not been established
- Geriatric Patients: In clinical trials the efficacy of rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years
- It is unknown if rivaroxaban is excreted in human milk. Discontinue drug or discontinue nursing.
-
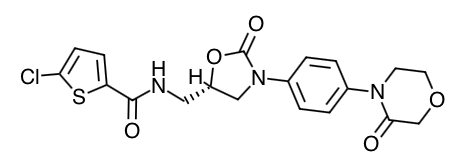
Scientific Name: 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide.
-
Empirical Formula: C19H18CIN3O5S
-
Molecular Weight: 435.89
- An oral factor Xa inhibitor that selectively blocks the active site of factor Xa and does not require a cofactor (such as Anti-thrombin III) for activity.
- Absorption:
- The absolute bioavailability of rivaroxaban is dose-dependent. For the 10 mg dose, it is estimated to be 80% to 100% and is not affected by food. Rivaroxaban 10 mg tablets can be taken with or without food. For the 20 mg dose in the fasted state the absolute bioavailability is approximately 66%.
- Coadministration of rivaroxaban with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39% and 76% respectively with food). Rivaroxaban 15 mg and 20 mg tablets should be taken with food.
- The maximum concentrations (Cmax) of rivaroxaban appear 2 to 4 hours after tablet intake.
- The pharmacokinetics of rivaroxaban were not affected by drugs altering gastric pH. Coadministration of rivaroxaban (30 mg single dose) with the H2-receptor antagonist ranitidine (150 mg twice daily), the antacid aluminum hydroxide/magnesium hydroxide (10mL) or rivaroxaban (20 mg single dose) with the PPI omeprazole (40 mg once daily) did not show an effect on the bioavailability and exposure of rivaroxaban.
- Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. A 29% and 56% decrease in AUC and Cmax compared to tablet was reported when rivaroxaban granulate is released in the proximal small intestine. Exposure is further reduced when drug is released in the distal small intestine, or ascending colon. Avoid administration of rivaroxaban distal to the stomach, which can result in reduced absorption and related drug exposure.
- In a study with 44 healthy subjects, both mean AUC and Cmax values for 20mg rivaroxaban administered orally as a crushed tablet mixed in applesauce were comparable to that after the whole tablet. However, for the crushed tablet, suspended in water and administered via an NG tube followed by a liquid meal, only mean AUC was comparable to that after the whole tablet, and Cmax was 18% lower.
- Distribution:
- Plasma protein binding of
rivaroxaban in human plasma is approximately 92% to 95%, with albumin being the
main binding component.
- The steady-state volume of distribution in healthy subjects is approximately 50 L.
- Metabolism:
- Approximately 51% of an orally administered [14C]-rivaroxaban dose was recovered as inactive metabolites in urine (30%) and feces (21%).
- Oxidative degradation catalyzed by CYP3A4/5 and CYP2J2 and hydrolysis are the major sites of biotransformation.
- Elimination:
- Following oral administration, approximately one-third of the absorbed dose is excreted unchanged in the urine, with the remaining two-thirds excreted as inactive metabolites in both the urine and feces.
- Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated Bcrp). Rivaroxaban's affinity for influx transporter proteins is unknown. Rivaroxaban is a low-clearance drug, with a systemic clearance of approximately 10L/hr in healthy volunteers following intravenous administration. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
- Specific Populations
- Gender: Gender did not influence the pharmacokinetics or pharmacodynamics of rivaroxaban.
- Race: Healthy Japanese subjects were found to have 20 to 40% on average, higher exposures compared to other ethnicities including Chinese. However, these differences in exposure are reduced when values are corrected for body weight.
- Elderly: In clinical studies, elderly subjects exhibited higher rivaroxaban plasma concentrations than younger subjects with mean AUC values being approximately 50% higher, mainly due to reduced (apparent) total body and renal clearance. Age related changes in renal function may play a role in this age effect. The terminal elimination half-life is 11 to 13 hours in the elderly.
- Body Weight: Extremes in body weight (<50 kg or >120 kg) did not influences (less than 25%) rivaroxaban exposure.
- Advise patients to take rivaroxaban only as directed and to not discontinue without first talking to their healthcare professional.
- Advise patients with atrial fibrillation to take rivaroxaban only daily with the evening meal.
- Advise patients with DVT and/or PE to take rivaroxaban 15 mg or 20 mg tablets with food at approximately the same time every day.
- Advise patients who cannot swallow the tablet whole to crush it and combine with a small amount of applesauce followed by food.
- For patients requiring an NG tube or gastric feeding tube, instruct the patient or caregiver to crush the rivaroxaban tablet and mix it with a small amount of water before administering via the tube.
- If a dose is missed, advise the patient to take rivaroxaban as soon as possible on the same day and continue on the following day with their recommended daily dose regimen.
- Advise patients to report any unusual bleeding or bruising to their physician. Inform patients that it might take them longer than usual to stop bleeding, and that they may bruise and/or bleed more easily when they are treated with rivaroxaban.
- If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur, advise the patient to contact his or her physician immediately.
- Advise patients to inform their healthcare professional that they are taking rivaroxaban before any invasive procedure (including dental procedures) is scheduled.
- Advise patients to inform their physicians and dentists if they are taking, or plan to take, any prescription or over-the-counter drugs or herbals.
- Drug Monograph: Apixaban (Eliquis)
- Drug Monograph: Abciximab (ReoPro)
Indications
Dosing
(Adult)
(Pediatrics)
Renal Dosing
Black Box Warnings
Warnings
Adverse Reactions
Overdose
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacokinetics
Counseling Points
Other EBM Consult Related Content
MESH Terms & Keywords
|
|---|
|