Pregabalin (Lyrica): Drug Monograph
|
|---|
- Neuropathic pain associated with diabetic peripheral neuropathy (DPN) and or spinal cord injury
- Post-herpetic neuralgia (PHN)
- Adjunctive therapy for adult patients with partial onset seizures
- Fibromyalgia
- General Dosing & Administration Notes:
- Given orally with or without food.
- When discontinuing pregabalin, taper gradually over a minimum of 1 week.
- Fibromyalgia:
- 75 mg by mouth twice daily
- May increase to 150 mg twice daily (300 mg/day) within 1 week if needed
- Maximum: 225 mg twice daily (450 mg/day)
- Neuropathic pain associated with diabetic peripheral neuropathy:
- 50 mg by mouth three times a day (150 mg/day)
- May increase to 300 mg/day within 1 week if needed
- Maximum: 100 mg three times a day (300 mg/day).
- Neuropathic pain associated with spinal cord injury:
- 75 mg by mouth twice daily
- May increase to 150 mg twice daily (300 mg/day) within 1 week if needed
- Maximum: 300 mg twice daily (600 mg/day) if no sufficient pain relief experienced following 2-3 weeks of treatment with 150 mg twice daily
- Partial onset seizures:
- Adjunctive therapy: 150 mg/day by mouth divided twice or three times a day
- May increase up to a maximum dose of 600 mg/day
- Postherpetic neuralgia:
- 75 mg by mouth twice a day (or) 50 mg three times a day (150 mg/day)
- May increase to 300 mg/day within 1 week if needed
- Maximum: 600 mg/day divided twice or three times a day if no sufficient pain relief experienced following 2-4 weeks of treatment with 300 mg/day
- If recommended dose of 150 mg/day given in two or three divided doses per day with normal renal function:
- CrCl 30-60 mL/min: 75 mg/day given in two or three divided doses per day
- CrCl 15-30 mL/min: 25-50 mg/day given in once or in two divided doses a day
- CrCl <15 mL/min: 25 mg/day every day
- If recommended dose of 300 mg/day given in two or three divided doses per day with normal renal function:
- CrCl 30-60 mL/min: 150 mg/day given in two to three divided doses a day
- CrCl 15-30 mL/min: 75 mg/day every day or in two divided doses a day
- CrCl <15 mL/min: 25-50 mg/day every day
- If recommended dose of 450 mg/day given in two or three divided doses per day with normal renal function:
- CrCl 30-60 mL/min: 225 mg/day given in two or three divided doses a day
- CrCl 15-30 mL/min: 100-150 mg/day every day or in two divided doses a day
- CrCl <15 mL/min: 50-75 mg/day every day
- If recommended dose of 600 mg/day given in two or three divided doses per day with normal renal function:
- CrCl 30-60 mL/min: 300 mg/day given in two or three divided doses a day
- CrCl 15-30 mL/min: 150 mg/day every day or two divided doses a day
- CrCl <15 mL/min: 75 mg/day every day
- Hemodialysis:
- 25 mg every day regimen: Take 1 supplemental dose of 25 mg or 50 mg
- 25-50 mg every day regimen: Take 1 supplemental dose of 50 mg or 75 mg
- 50-75 mg every day regimen: Take 1 supplemental dose of 75 or 100 mg
- 75 mg every day regimen: Take 1 supplemental dose of 100 mg or 150 mg
- Discontinuation:
- Taper over minimum of 1 week
- Capsules: 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, 300 mg
- Solution: 20 mg/mL [16 fl oz (or) 473 mL]
- Angioedema may be associated with life-threatening respiratory compromise requiring emergency treatment. Discontinue pregabalin immediately.
- Hypersensitivity reactions, such as hives, dyspnea, and wheezing.
- Increased seizure frequency ay occur in patients with seizure disorders if pregabalin is rapidly discontinued.
- Risk of suicidal thoughts or behaviors.
- Peripheral edema
- Dizziness and somnolence. Impaired ability to drive or operate machinery.
- Weight gain
- Tumorigenic potential
- Ophthalmological effects
- Creatine Kinase elevations
- Decreased platelet count
- PR Interval prolongation
- Dizziness
- Somnolence
- Dry mouth
- Edema
- Blurred vision
- Weight gain
- Thinking abnormal (primarily difficulty with concentration/attention)
- Elimination of unabsorbed drug may be attempted by emesis or gastric lavage.
- General supportive care of the patient is indicated.
- Standard hemodialysis procedures result in significant clearance of pregabalin (approximately 50% in 4 hours).
- Pregabalin is unlikely to be involved in significant pharmacokinetic drug interactions.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: The effects are unknown.
- Nursing Mothers: It is not known if pregabalin is excreted in human milk. Decision should be made to discontinue nursing or to discontinue the drug.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: The safety and efficacy of pregabalin in pediatric patients have not been established.
- Geriatric Patients: No overall differences in safety and efficacy observed between elderly and younger patients. Adjustment for elderly patients with renal impairment necessary.
- It is not known if pregabalin is excreted in human milk. Decision should be made to discontinue nursing or to discontinue the drug.
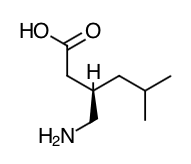
- Scientific Name: (S)-3-(aminonethyl)-5-methylhexanoic acid.
- Empirical Formula: C8H17NO2
- Molecular Weight: 159.23
- Pregabalin binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin's anti-nociceptive and antiseizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium- dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord.
- While pregabalin is a structural derivative of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), it does not bind directly to GABA A, GABA B, or benzodiazepine receptors, does not augment GABA A responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake.
- Pregabalin is well absorbed after oral administration, is eliminated largely by renal excretion, and has an elimination half-life of about 6 hours.
- Absorption: Following oral administration of pregabalin capsules under fasting conditions, peak plasma concentrations occur within 1.5 hours. Pregabalin oral bioavailability is ≥90% and is independent of dose. Following single- (25 to 300 mg) and multiple- dose (75 to 900 mg/day) administration, maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) values increase linearly. Following repeated administration, steady state is achieved within 24 to 48 hours. Multiple-dose pharmacokinetics can be predicted from single-dose data. The rate of pregabalin absorption is decreased when given with food, resulting in a decrease in Cmax of approximately 25% to 30% and an increase in Tmax to approximately 3 hours. However, administration of pregabalin with food has no clinically relevant effect on the total absorption of pregabalin. Therefore, pregabalin can be taken with or without food.
- Distribution: Pregabalin does not bind to plasma proteins. The apparent volume of distribution of pregabalin following oral administration is approximately 0.5 L/kg. Pregabalin is a substrate for system L transporter which is responsible for the transport of large amino acids across the blood brain barrier. Although there are no data in humans, pregabalin has been shown to cross the blood brain barrier in mice, rats, and monkeys. In addition, pregabalin has been shown to cross the placenta in rats and is present in the milk of lactating rats.
- Metabolism: Pregabalin undergoes negligible metabolism in humans. Following a dose of radiolabeled pregabalin, approximately 90% of the administered dose was recovered in the urine as unchanged pregabalin. The N-methylated derivative of pregabalin, the major metabolite of pregabalin found in urine, accounted for 0.9% of the dose. In preclinical studies, pregabalin (S- enantiomer) did not undergo racemization to the R-enantiomer in mice, rats, rabbits, or monkeys.
- Elimination: Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug with a mean elimination half-life of 6.3 hours in subjects with normal renal function. Mean renal clearance was estimated to be 67.0 to 80.9 mL/min in young healthy subjects. Because pregabalin is not bound to plasma proteins this clearance rate indicates that renal tubular reabsorption is involved. Pregabalin elimination is nearly proportional to creatinine clearance (CLCr).
- Special Populations
- Race: In population pharmacokinetic analyses of the clinical studies in various populations, the pharmacokinetics of pregabalin were not significantly affected by race (Caucasians, Blacks, and Hispanics).
- Gender: Population pharmacokinetic analyses of the clinical studies showed that the relationship between daily dose and pregabalin drug exposure is similar between genders.
- Renal Impairment and Hemodialysis: Pregabalin clearance is nearly proportional to creatinine clearance (CLCr). Dosage reduction in patients with renal dysfunction is necessary. Pregabalin is effectively removed from plasma by hemodialysis. Following a 4-hour hemodialysis treatment, plasma pregabalin concentrations are reduced by approximately 50%. For patients on hemodialysis, dosing must be modified.
- Elderly: Pregabalin oral clearance tended to decrease with increasing age. This decrease in pregabalin oral clearance is consistent with age-related decreases in CLCr. Reduction of pregabalin dose may be required in patients who have age-related compromised renal function.
- Pediatric Pharmacokinetics: Pharmacokinetics of pregabalin have not been adequately studied in pediatric patients.
- Drug Interactions
- In Vitro Studies: Pregabalin, at concentrations that were, in general, 10-times those attained in clinical trials, does not inhibit human CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 enzyme systems. In vitro drug interaction studies demonstrate that pregabalin does not induce CYP1A2 or CYP3A4 activity. Therefore, an increase in the metabolism of coadministered CYP1A2 substrates (e.g. theophylline, caffeine) or CYP 3A4 substrates (e.g., midazolam, testosterone) is not anticipated.
- In Vivo Studies: The drug interaction studies described in this section were conducted in healthy adults, and across various patient populations.
- Gabapentin: The pharmacokinetic interactions of pregabalin and gabapentin were investigated in 12 healthy subjects following concomitant single-dose administration of 100-mg pregabalin and 300-mg gabapentin and in 18 healthy subjects following concomitant multiple-dose administration of 200-mg pregabalin every 8 hours and 400-mg gabapentin every 8 hours. Gabapentin pharmacokinetics following single- and multiple-dose administration were unaltered by pregabalin coadministration. The extent of pregabalin absorption was unaffected by gabapentin coadministration, although there was a small reduction in rate of absorption.
- Oral Contraceptive: Pregabalin co-administration (200 mg three times a day) had no effect on the steady-state pharmacokinetics of norethindrone and ethinyl estradiol (1 mg/35μg, respectively) in healthy subjects.
- Lorazepam: Multiple-dose administration of pregabalin (300 mg twice a day) in healthy subjects had no effect on the rate and extent of lorazepam single-dose pharmacokinetics and single-dose administration of lorazepam (1 mg) had no effect on the steady-state pharmacokinetics of pregabalin.
- Oxycodone: Multiple-dose administration of pregabalin (300 mg twice a day) in healthy subjects had no effect on the rate and extent of oxycodone single-dose pharmacokinetics. Single-dose administration of oxycodone (10 mg) had no effect on the steady-state pharmacokinetics of pregabalin.
- Ethanol: Multiple-dose administration of pregabalin (300 mg twice a day) in healthy subjects had no effect on the rate and extent of ethanol single-dose pharmacokinetics and single-dose administration of ethanol (0.7 g/kg) had no effect on the steady-state pharmacokinetics of pregabalin.
- Phenytoin, carbamazepine, valproic acid, and lamotrigine: Steady-state trough plasma concentrations of phenytoin, carbamazepine and carbamazepine 10,11 epoxide, valproic acid, and lamotrigine were not affected by concomitant pregabalin (200 mg three times a day) administration
- Instruct patients to take pregabalin only as prescribed. Abrupt or rapid discontinuation may result in insomnia, nausea, headache anxiety, hyperhidrosis, or diarrhea.
- Advise patients that pregabalin may cause angioedema, with swelling of the face, mouth (lip, gum, tongue) and neck (larynx and pharynx) that can lead to life-threatening respiratory compromise. Discontinue pregabalin and immediately seek medical care if they experience symptoms.
- Advise patients that pregabalin has been associated with hypersensitivity reactions such as wheezing, dyspnea, rash, hives and blisters. Discontinue and immediately seek medical care if they experience these symptoms.
- Pregabalin may increase the risk of suicidal thoughts and behaviors. Be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior or thoughts about self-harm. Report behaviors of concern immediately to health care providers.
- Tell patients that pregabalin may cause dizziness, somnolence, blurred vision and other CNS signs and symptoms. Advise patients not to drive, operate complex machinery, or engage in other hazardous activities until they have gained sufficient experience on pregabalin to gauge whether or not if affects their mental, visual, and/or motor performance adversely.
- Counsel patients that pregabalin may cause edema and weight gain.
- Counsel patients that pregabalin may cause visual disturbances. If changes in their vision occur, they should notify their physician.
- Instruct patients to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever.
- Inform patients who require concomitant treatment with central nervous system depressants such as opiates or benzodiazepines that they may experience additive CNS side effects, such as somnolence.
- Tell patients to avoid consuming alcohol while taking pregabalin.
- Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy.
- Instruct patients to notify their physician if they are breast-feeding or intend to breast feed during therapy.
- Inform men being treated with pregabalin who plan to father a child of the potential risk of male-mediated teratogenicity.
- Instruct diabetic patients to pay particular attention to skin integrity while being treated with pregabalin.
Indications
Dosing (Adult)
Renal Dosing
Dosage Forms
Warnings
Adverse Reactions
Overdose
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|