Linezolid (Zyvox): Drug Monograph
|
|---|
- Community-acquired pneumonia, including cases with concurrent bacteremia,
- Complicated skin and skin structure infections, including diabetic foot infections, without concomitant osteomyelitis. Linezolid has not been studied in the treatment of decubitus ulcers.
- Nosocomial pneumonia
- Uncomplicated skin and skin structure infection
- Vancomycin-resistant Enterococcus faecium infections, including cases with concurrent bacteremia
- Infections caused by susceptible strains of designated microorgamisms
- The safety and efficacy of linezolid formulations given for longer than 28 days have not been evaluated in controlled clinical trials
- The oral and IV doses are the same due to oral bioavailability being 100%
- Oral: May be administered without regard to the timing of meals
- IV: Infuse over 30-120 minutes
-
Pneumonia, nosocomial/ community-acquired pneumonia, including concurrent bacteremia:
- 600 mg IV or orally every 12 hours x 10-14 days
- If MRSA pneumonia may need treatment up to 21 days
- Skin and skin structure infections:
- Complicated: 600 mg IV or orally every 12 hours x 10-14 consecutive days
- If diabetic foot infection, treatment duration can be up to 4 weeks
- Uncomplicated: 400 mg IV or orally every 12 hours x 10-14 consecutive days
-
Vancomycin-resistantEnterococcus faecium infections, including concurrent bacteremia:
- 600 mg intravenous or oral every 12 hours for 14-28 days
-
Pneumonia, nosocomial/community-acquired pneumonia, including concurrent bacteremia:
- Preterm neonates <7 days of age:
- 10 mg/kg IV or orally every 12 hours. Increase to 10 mg/kg every 8 hours if clinical response is suboptimal. All neonates should receive 10 mg/kg every 8 hours by 7 days of life. Treat for 10-14 consecutive days.
- Birth-11 years:
- 10 mg/kg IV or orally every 8 hours for 10-14 consecutive days
- ≥12 years:
- 600 mg IV or orally every 12 hours for 10-14 consecutive days
-
Vancomycin-resistantEnterococcus faecium infections, including concurrent bacteremia:
- Neonates <7 days of age:
- 10 mg/kg IV or orally every 12 hours. Increase to 10 mg/kg every 8 hours if clinical response is suboptimal.
- All neonates should receive 10 mg/kg every 8 hours by 7 days of life.
- Treat for 14-28 consecutive days
- Birth-11 years:
- 10 mg/kg IV or orally every 8 hours for 14-28 consecutive days
- ≥12 years: 600 mg IV or orally every 12 hours for 14-28 consecutive days
- Skin and skin structure infections (Complicated):
- Preterm neonates <7 days of age:
- 10 mg/kg IV or orally every 12 hours. Increase to 10 mg/kg every 8 hours if clinical response is suboptimal. All neonates should receive 10 mg/kg every 8 hours by 7 days of life. Treat for 10-14 consecutive days.
- Birth-11 years, injection, suspension, or tablet: 10 mg/kg every 8 hours for 10-14 consecutive days
- ≥12 years, injection suspension, or tablet: 600 mg every 12 hours for 10-14 consecutive days
- Skin and skin structure infections (Uncomplicated):
- Neonates <7 days of age, suspension: 10 mg/kg every 12 hours. Increase to 10 mg/kg every 8 hours if clinical response is suboptimal. All neonates should receive 10 mg/kg every 8 hours by 7 days of life. Treat for 10-14 consecutive days
- <5 years, suspension or tablet: 10 mg/kg every 8 hours for 10-14 consecutive days
- 5-11 years, suspension or tablet: 10 mg/kg every 12 hours for 10-14 consecutive days
- ≥12 years, suspension or tablet: 600 mg every 12 hours for 10-14 consecutive days
- None
- Hemodialysis:
- After a 3 hour session about 30% is removed; consider giving additional dosing if the time to next dose after dialysis is not close to immediate.
- Injection: 2 mg/mL (100 mL, 200 mL, 300 mL)
- Tablet: 600 mg
- Oral suspension: 100 mg per each 5 mL
- Known hypersensitivity to linezolid or any of the other product components.
- Patients taking any monoamine oxidase inhibitor (MAOI) or within two weeks of taking an MAOI.
- Myelosuppression: Monitor complete blood counts weekly. Consider discontinuation in patients who develop or have worsening myelosuppression.
- Peripheral and optic neuropathy: Reported primarily in patients treated for longer than 28 days. If patients experience symptoms of visual impairment, prompt ophthalmic evaluation is recommended.
- Serotonin syndrome: Patients taking serotonergic antidepressants should receive ZYVOX only if no other therapies are available. Discontinue serotonergic antidepressants and monitor patients for signs and symptoms of both serotonin syndrome and antidepressant discontinuation.
- A mortality imbalance was seen in an investigational study in linezolid-treated patients with catheter-related bloodstream infections.
- Clostridium difficile associated diarrhea: Evaluate if diarrhea occurs.
- Potential interactions producing elevation of blood pressure: monitor blood pressure
- Hypoglycemia: Postmarketing cases of symptomatic hypoglycemia have been reported in patients with diabetes mellitus receiving insulin or oral hypoglycemic agents.
- Lactic acidosis: Should receive immediate medical evaluation for recurrent nausea or vomiting, unexplained acidosis, or a low bicarbonate level.
- Convulsions
- Development of drug-resistant bacteria
- Supportive care is advised, with maintenance of glomerular filtration.
- Hemodialysis may facilitate more rapid elimination of linezolid.
- No data for removal with peritoneal dialysis or hemoperfusion.
- Clinical signs may include decreased activity, ataxia, vomiting, and tremors (data from animal studies.)
- Inhibits: Monoamine oxidase (MAO) enzyme
- Adrenergic and serotonergic agents - potential for interaction
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether linezolid is excreted in human milk. Caution should be exercised when administered to a nursing woman.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: The safety and effectiveness of linezolid for the treatment of pediatric patients has been established for: nosocomial pneumonia, complicated skin and skin structure infections, community-acquired pneumonia, vancomycin-resistant Enterococcus faecium infections, ad uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes.
- Geriatric Patients: No overall differences in safety or effectiveness were observed between older and younger patients. Greater sensitivity of some older individuals cannot be ruled out.
- It is not known whether linezolid is excreted in human milk. Caution should be exercised when administered to a nursing woman.
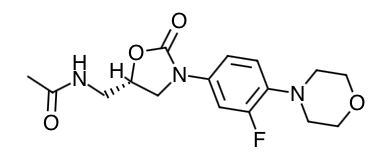
- Scientific Name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide
- Empirical Formula: C16H20FN3O4
- Molecular Weight: 337.35
- Linezolid is an antibacterial drug that inhibits bacterial protein synthesis by inhibiting bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initial complex, which is an essential component of the bacterial translation process.
- In a randomized, positive- and placebo-controlled crossover thorough QT study, 40 healthy subjects were administered a single linezolid 600 mg dose via a 1 hour IV infusion, a single linezolid 1200 mg dose via a 1 hour IV infusion, placebo, and a single oral dose of positive control. At both the 600 mg and 1200 mg linezolid doses, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
- Absorption:
- Linezolid is extensively absorbed after oral dosing with bioavailability of 100%
- Maximum plasma concentrations are reached approximately 1 to 2 hours after dosing
- Linezolid may be administered without regard to the timing of meals.
- The time to reach the maximum concentration is delayed from 1.5 hours to 2.2 hours and Cmax is decreased by about 17% when high fat food is given with linezolid.
- However, the total exposure measured as AUC 0-∞ is similar under both conditions.
- Distribution:
- The volume of distribution of linezolid at steady-state averaged 40 to 50 liters
- The ratio of linezolid in saliva relative to plasma was 1.2 to 1 and the ratio of linezolid in sweat relative to plasma was 0.55 to 1.
- Protein Binding:
- About 31% and is concentration-independent
- Metabolism:
- Linezolid is primarily metabolized by oxidation of the morpholine ring, which results in two inactive ring-opened carboxylic acid metabolites: the aminoethoxyacetic acid metabolite (A), and the hydroxyethyl glycine metabolite (B). Formation of metabolite A is presumed to be formed via an enzymatic pathway whereas metabolite B is mediated by a non-enzymatic chemical oxidation mechanism in vitro.
- In vitro studies have demonstrated that linezolid is minimally metabolized and may be mediated by human cytochrome P450. However, the metabolic pathway of linezolid is not fully understood.
- Elimination:
- Nonrenal clearance accounts for approximately 65% of the total clearance of linezolid.
- The mean renal clearance of linezolid is 40 mL/min which suggests net tubular reabsorption.
- Virtually no linezolid appears in the feces, while approximately 6% of the dose appears in the feces as metabolite B, and 3% as metabolite A.
- Specific Populations
- Geriatric Patients: The pharmacokinetics of linezolid are not significantly altered in elderly patients (65 years or older). Therefore, dose adjustment for geriatric patients is not necessary.
- Pediatric Patients: The pharmacokinetics of linezolid following a single intravenous dose were investigated in pediatric patients ranging in age from birth through 17 years (including premature and full-term neonates), in healthy adolescent subjects ranging in age from 12 through 17 years, and in pediatric patients ranging in age from 1 week through 12 years. The Cmax and the volume of distribution (Vss) of linezolid are similar regardless of age in pediatric patients. However, plasma clearance of linezolid varies as a function of age. With the exclusion of pre-term neonates less than one week of age, weight-based clearance is most rapid in the youngest age groups ranging from < 1 week old to 11 years, resulting in lower single-dose systemic exposure (AUC) and a shorter half-life as compared with adults. As the age of pediatric patients increases, the weight-based clearance of linezolid gradually decreases, and by adolescence, mean clearance values approach those observed for the adult population. There is increased inter-subject variability in linezolid clearance and systemic drug exposure (AUC) across all pediatric age groups as compared with adults. Similar mean daily AUC values were observed in pediatric patients from birth to 11 years of age dosed every 8 hours relative to adolescents or adults dosed every 12 hours. Therefore, the dosage for pediatric patients up to 11 years of age should be 10 mg/kg every 8 hours. Pediatric patients 12 years and older should receive 600 mg every 12 hours.
- Gender: Females have a slightly lower volume of distribution of linezolid than males. Plasma concentrations are higher in females than in males, which is partly due to body weight differences. After a 600-mg dose, mean oral clearance is approximately 38% lower in females than in males. However, there are no significant gender differences in mean apparent elimination-rate constant or half-life. Thus, drug exposure in females is not expected to substantially increase beyond levels known to be well tolerated. Therefore, dose adjustment by gender does not appear to be necessary.
- Renal Impairment: The pharmacokinetics of the parent drug, linezolid, are not altered in patients with any degree of renal impairment; however, the two primary metabolites of linezolid accumulate in patients with renal impairment, with the amount of accumulation increasing with the severity of renal dysfunction. The pharmacokinetics of linezolid and its two metabolites have also been studied in patients with end-stage renal disease (ESRD) receiving hemodialysis. In the ESRD study, 14 patients were dosed with linezolid 600 mg every 12 hours for 14.5 days. Because similar plasma concentrations of linezolid are achieved regardless of renal function, no dose adjustment is recommended for patients with renal impairment. However, given the absence of information on the clinical significance of accumulation of the primary metabolites, use of linezolid in patients with renal impairment should be weighed against the potential risks of accumulation of these metabolites. Both linezolid and the two metabolites are eliminated by hemodialysis. No information is available on the effect of peritoneal dialysis on the pharmacokinetics of linezolid. Approximately 30% of a dose was eliminated in a 3-hour hemodialysis session beginning 3 hours after the dose of linezolid was administered; therefore, linezolid should be given after hemodialysis.
- Hepatic Impairment: The pharmacokinetics of linezolid are not altered in patients (n=7) with mild-to-moderate hepatic impairment (Child-Pugh class A or B). On the basis of the available information, no dose adjustment is recommended for patients with mild-to-moderate hepatic impairment. The pharmacokinetics of linezolid in patients with severe hepatic impairment have not been evaluated.
- Drug Interactions
- Drugs Metabolized by Cytochrome P450: Linezolid is not an inducer of cytochrome P450 (CYP450) in rats. In addition, linezolid does not inhibit the activities of clinically significant human CYP isoforms (e.g., 1A2, 2C9, 2C19, 2D6, 2E1, 3A4). Therefore, linezolid is not expected to affect the pharmacokinetics of other drugs metabolized by these major enzymes.
- Antibiotics
- Aztreonam: The pharmacokinetics of linezolid or aztreonam are not altered when administered together.
- Gentamicin: The pharmacokinetics of linezolid or gentamicin are not altered when administered together.
- Antioxidants: The potential for drug-drug interactions with linezolid and the antioxidants Vitamin C and Vitamin E was studied in healthy volunteers. Subjects were administered a 600 mg oral dose of linezolid on Day 1, and another 600 mg dose of linezolid on Day 8. On Days 2-9, subjects were given either Vitamin C (1000 mg/day) or Vitamin E (800 IU/ day). The AUC 0-∞ of linezolid increased 2.3% when co-administered with Vitamin C and 10.9% when co-administered with Vitamin E. No linezolid dose adjustment is recommended during co-administration with Vitamin C or Vitamin E.
- Strong CYP 3A4 Inducers
- Rifampin: The effect of rifampin on the pharmacokinetics of linezolid was evaluated in a study of 16 healthy adult males. Volunteers were administered oral linezolid 600 mg twice daily for 5 doses with and without rifampin 600 mg once daily for 8 days. Co-administration of rifampin with linezolid resulted in a 21% decrease in linezolid Cmax [90% CI, 15% - 27%] and a 32% decrease in linezolid AUC 0-12 [90% CI, 27% - 37%]. The clinical significance of this interaction is unknown. The mechanism of this interaction is not fully understood and may be related to the induction of hepatic enzymes. Other strong inducers of hepatic enzymes (e.g. carbamazepine, phenytoin, phenobarbital) could cause a similar or smaller decrease in linezolid exposure.
- Monoamine Oxidase Inhibition: Linezolid is a reversible, nonselective inhibitor of monoamine oxidase. Therefore, linezolid has the potential for interaction with adrenergic and serotonergic agents.
- Adrenergic Agents: Some individuals receiving linezolid may experience a reversible enhancement of the pressor response to indirect-acting sympathomimetic agents, vasopressor or dopaminergic agents. Commonly used drugs such as phenylpropanolamine and pseudoephedrine have been specifically studied. Initial doses of adrenergic agents, such as dopamine or epinephrine, should be reduced and titrated to achieve the desired response.
- Tyramine: A significant pressor response has been observed in normal adult subjects receiving linezolid and tyramine doses of more than 100 mg. Therefore, patients receiving linezolid need to avoid consuming large amounts of foods or beverages with high tyramine content.
- Pseudoephedrine HCl or phenylpropanolamine HCl: A reversible enhancement of the pressor response of either pseudoephedrine HCl (PSE) or phenylpropanolamine HCl (PPA) is observed when linezolid is administered to healthy normotensive subjects. A similar study has not been conducted in hypertensive patients. The interaction studies conducted in normotensive subjects evaluated the blood pressure and heart rate effects of placebo, PPA or PSE alone, linezolid alone, and the combination of steady-state linezolid (600 mg every 12 hours for 3 days) with two doses of PPA (25 mg) or PSE (60 mg) given 4 hours apart. Heart rate was not affected by any of the treatments. Blood pressure was increased with both combination treatments. Maximum blood pressure levels were seen 2 to 3 hours after the second dose of PPA or PSE, and returned to baseline 2 to 3 hours after peak. The results of the PPA study follow, showing the mean (and range) maximum systolic blood pressure in mm Hg: placebo = 121 (103 to 158); linezolid alone = 120 (107 to 135); PPA alone = 125 (106 to 139); PPA with linezolid = 147 (129 to 176). The results from the PSE study were similar to those in the PPA study. The mean maximum increase in systolic blood pressure over baseline was 32 mm Hg (range: 20-52 mm Hg) and 38 mm Hg (range: 18-79 mm Hg) during co-administration of linezolid with pseudoephedrine or phenylpropanolamine, respectively.
- Serotonergic Agents
- Dextromethorphan: The potential drug-drug interaction with dextromethorphan was studied in healthy volunteers. Subjects were administered dextromethorphan (two 20-mg doses given 4 hours apart) with or without linezolid. No serotonin syndrome effects (confusion, delirium, restlessness, tremors, blushing, diaphoresis, hyperpyrexia) have been observed in normal subjects receiving linezolid and dextromethorphan.
- Patients should be counseled that antibacterial drugs, including linezolid, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold).
- Patients should be advised to take the medication exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by linezolid or other antibacterial drugs in the future.
- Advise patients that the drug may be taken with or without food.
- Patients should be advised to inform their physician if they have a history of hypertension.
- Patients should be advised to avoid large quantities of foods or beverages with high tyramine content during treatment. Foods high in tyramine content include those that may have undergone protein changes by aging, fermentation pickling or smoking to improve flavor, such as aged cheeses, fermented or air-dried meats, sauerkraut soy sauce, tap beers, and red wines. The tyramine content of any protein-rich food may be increased if stored for long periods or improperly refrigerated.
- Patients should be advised to inform their physician of any medication they are taking. These include medications containing pseudoephedrine HCl of phenylpropanolamine HCl, such as cold remedies and decongestants and serotonin re-uptake inhibitors or other antidepressants.
- Each 5 mL of the 100 mg/5mL of the oral suspension contains 20 mg phenylalanine. The other formulations do not contain phenylalanine.
- Patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
- Patients should be advised to inform their physician if they experience changes in vision.
- Patients should be advised to inform their physician if they have a history of seizures
- Inform patients particularly those with diabetes mellitus, that hypoglycemic reactions, such as diaphoresis and tremulousness, along with low blood glucose measurements may occur when treated with linezolid. If such reactions occur patients should contact a physician or other health professional for proper treatment.
Indications
Dosing
(Adult):
General Notes:
(Pediatrics):
Renal Dosing
Dosage Forms
Contraindications
Warnings
Overdose
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|

