Carvedilol (Coreg): Drug Monograph
|
|---|
- Treatment of mild to severe chronic heart failure
- Treatment of left ventricular dysfunction following myocardial infarction in clinically stable patients
- Treatment of hypertension
- General Dosing Considerations:
- Take with food
- The doses between the immediate-release (IR) formulation and controlled-release (CR) are not the same or dose-for-dose interchangeable (higher doses of the CR are needed to be the same as IR.
- IR 3.125 mg taken twice a day = CR 10 mg once daily
- IR 6.25 mg taken twice a day = CR 20 mg once daily
- IR 12.5 mg taken twice a day = CR 40 mg once daily
- IR 25 mg taken twice a day = CR 80 mg once daily
- Reduce dose if pulse starts to decrease to < 55 beats per minute
- Heart failure:
- Initiate at low dose and titrate slow:
- Note: Symptoms of heart failure can initially worsen with each dose titration and last about 7-10 days. Starting a lower doses and titrating slow can help minimize these effects
- Immediate Release Formulation: start at 3.25 mg twice daily and increase to 6.25, 12.5, and then 25 mg twice daily over intervals of at least 2 weeks. If > 85 kg, then titrate up to 50 mg twice daily.
- Controlled Release Formulation: 10 mg once daily x 2 weeks, then increase to 20 mg, 40 mg, and 80 mg at 2 week intervals and as tolerated to max dose of 80 mg daily.
- Hypertension:
- Immediate Release: 6.25 mg twice daily and increase if needed for blood pressure control to 12.5 mg then 25 mg twice daily over intervals of 1 to 2 weeks.
- Controlled Release: 20 mg once daily and increase every 1-2 weeks as tolerate to 40 and then 80 mg once a day.
- Left ventricular dysfunction following myocardial infarction:
- Initiate at low dose and titrate slow:
- Note: Symptoms of heart failure can initially worsen with each dose titration and last about 7-10 days. Starting a lower doses and titrating slow can help minimize these effects
- Immediate Release Formulation: start at 3.25 mg twice daily and increase to 6.25, 12.5, and then 25 mg twice daily over intervals of at least 2 weeks. If > 85 kg, then titrate up to 50 mg twice daily.
- Controlled Release Formulation: 10 mg once daily x 2 weeks, then increase to 20 mg, 40 mg, and 80 mg at 2 week intervals and as tolerated to max dose of 80 mg daily.
- None
- Tablets (Immediate Release): 3.125, 6.25, 12.5, 25 mg
- Capsules (Controlled Release 24 hr; as Coreg CR): 10, 20, 40, 80 mg
- Bronchial asthma or related bronchospastic conditions
- Second- or third-degree AV block
- Sick sinus syndrome
- Severe bradycardia (unless permanent pacemaker in place)
- Patients in cardiogenic shock or decompensated heart failure requiring the use of IV inotropic therapy
- Severe hepatic impairment
- History of serious hypersensitivity reaction
- Acute exacerbation of coronary artery disease upon cessation of therapy; do not abruptly discontinue.
- Bradycardia, hypotension, worsening heart failure/fluid retention may occur. Reduce dose as needed.
- Non-allergic bronchospasm (e.g., chronic bronchitis and emphysema): Avoid β-blockers. If deemed necessary, use with caution and at lowest effective dose.
- Diabetes: monitor glucose as beta-blockers may mask symptoms of hypoglycemia or worsen hyperglycemia.
- Peripheral vascular disease - may precipitate or aggravate symptoms of arterial insufficiency
- Deterioration of renal function - patients with low blood pressure, ischemic heart disease and diffuse vascular disease, and/or underlying renal insufficiency appear to be at risk.
- Anesthesia and major surgery - care should be taken when anesthetic agents, which depress myocardial function, such as ether, cyclopropane, and trichloroethylene, are used.
- Thyrotoxicosis
- Pheochromocytoma
- Prinzmetal's variant angina
- Risk of anaphylactic reaction
- Heart failure and left ventricular dysfunction following myocardial infarction (≥10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, asthenia, bradycardia, weight increase
- Hypertension (≥5%): Dizziness
- Severe hypotension, bradycardia, cardiac insufficiency, cardiogenic shock, and cardiac arrest may result as well as respiratory problems, bronchospasms, vomiting, lapses of consciousness, and generalized seizures.
- Place patient in supine position and, where necessary, keep under observation and treat under intensive-care conditions.
- Gastric lavage or pharmacologically induced emesis may be used shortly after ingestion. The following agents may be administered:
- For excessive bradycardia: Atropine, 2 mg IV.
- To support cardiovascular function: Glucagon, 5 to 10 mg IV rapidly over 30 seconds, followed by a continuous infusion of 5 mg/hour; sympathomimetics (dobutamine, isoprenaline, adrenaline) at doses according to body weight and effect.
- If peripheral vasodilation dominates, it may be necessary to administer adrenaline or noradrenaline with continuous monitoring of circulatory conditions. For therapy-resistant bradycardia, pacemaker therapy should be performed. For bronchospasm, β-sympathomimetics (as aerosol or IV) or aminophylline IV should be given.
- In the event of seizures, slow IV injection of diazepam or clonazepam is recommended. In the event of severe intoxication where there are symptoms of shock, treatment with antidotes must be continued for a sufficiently long period of time consistent with the 7- to 10-hour half-life of carvedilol.
- Atropine (1-2 mg IV), and/or glucagon (3 to 10 mg IV over 3 to 5 minutes, followed by a continuous infusion of 1 to 5 mg/hour)
- Sympathomimetics (dobutamine, isoprenaline, adrenaline) at doses according to body weight and effect
- Substrate: CYP2D6 (major), 1A2, 2C9, 2E1 and 3A4 are all minor. P-glycoprotein
- Inhibitor: None
- Inducer: None
- Considerations:
- CYP P450 2D6 enzyme inhibitors may increase and rifampin may decrease carvedilol levels. Hypotensive agents (e.g., reserpine, MAO inhibitors, clonidine) may increase the risk of hypotension and/or severe bradycardia.
- Cyclosporine or digoxin levels may increase.
- Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
- Amiodarone may increase carvedilol levels resulting in further slowing of the heart rate or cardiac conduction.
- Verapamil- or diltiazem-type calcium channel blockers may affect ECG and/or blood pressure.
- Insulin and oral hypoglycemics action may be enhanced.
- Pregnancy: Pregnancy Category C
- Labor and Delivery: None
- Nursing Mothers: It is not known whether this drug is excreted in human milk. Decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: Effectiveness in patients younger than 18 years of age has not been established.
- Geriatric Patients: With the exception of dizziness in hypertensive patients (incidence 8.8% in the elderly versus 6% in younger patients), no overall differences in the safety of effectiveness were observed between older and younger subjects.
- It is not known whether this drug is excreted in human milk. Decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
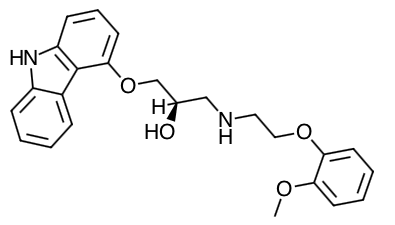
Scientific Name: (±)-1-(Carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol
-
Empirical Formula: C24H26N2O4
-
Molecular Weight: 406.5
- Carvedilol is a racemic mixture that is known to have nonselective beta-adrenoreceptor blocking activity due to the S(-) enantiomer and alpha-1-adrenergic blocking activity due to both R(+) and S(-) enantiomers.
- Carvedilol has no intrinsic sympathomimetic activity.
- Heart failure: The basis for the beneficial effects of carvedilol in heart failure is not established. Two placebo-controlled studies compared the acute hemodynamic effects of carvedilol to baseline measurements in 59 and 49 patients with NYHA class II-IV heart failure receiving diuretics, ACE inhibitors, and digitalis. There were significant reductions in systemic blood pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, and heart rate. Initial effects on cardiac output, stroke volume index, and systemic vascular resistance were small and variable.
- Hypertension: The mechanism by which alpha-blockade produces an antihypertensive effect has not been established. Beta-adrenoreceptor blocking activity has been demonstrated in animal and human studies showing that carvedilol (1) reduces cardiac output in normal subjects; (2) reduces exercise- and/or isoproterenol-induced tachycardia; and (3) reduces reflex orthostatic tachycardia. a1- adrenoreceptor blocking activity has been demonstrated in human and animal studies, showing that carvedilol (1) attenuates the pressor effects of phenylephrine; (2) causes vasodilation; and (3) reduces peripheral vascular resistance. These effects contribute to the reduction of blood pressure and usually are seen within 30 minutes of drug administration.
- Due to the alpha-1-receptor blocking activity of carvedilol, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension (1.8%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when carvedilol is administered with food at the recommended starting dose and titration increments are closely followed.
- In hypertensive patients with normal renal function, therapeutic doses of carvedilol decreased renal vascular resistance with no change in glomerular filtration rate or renal plasma flow. Changes in excretion of sodium, potassium, uric acid, and phosphorus in hypertensive patients with normal renal function were similar after carvedilol and placebo.
- Carvedilol has little effect on plasma catecholamines, plasma aldosterone, or electrolyte levels, but it does significantly reduce plasma renin activity when given for at least 4 weeks. It also increases levels of atrial natriuretic peptide.
- Absorption: Absolute bioavailability of ~25% to 35% due to a significant degree of first-pass metabolism.
- When administered with food, the rate of absorption is slowed, as evidenced by a delay in the time to reach peak plasma levels, with no significant difference in extent of bioavailability.
- Distribution: It is a lipophilic compound with a volume of distribution of approximately 115 L.
- Protein Binding: More than 98% bound to plasma proteins, primarily with albumin. The plasma-protein binding is independent of concentration over the therapeutic range.
- Metabolism:
- Carvedilol is extensively metabolized. Following oral administration of radiolabelled carvedilol to healthy volunteers, carvedilol accounted for only about 7% of the total radioactivity in plasma as measured by area under the curve (AUC). Less than 2% of the dose was excreted unchanged in the urine. Carvedilol is metabolized primarily by aromatic ring oxidation and glucuronidation. The oxidative metabolites are further metabolized by conjugation via glucuronidation and sulfation. The metabolites of carvedilol are excreted primarily via the bile into the feces.
- Compared to carvedilol, the 3 active metabolites exhibit weak vasodilating activity. Plasma concentrations of the active metabolites are about one-tenth of those observed for carvedilol and have pharmacokinetics similar to the parent.
- The primary P450 enzymes responsible for the metabolism of both R(+) and S(-)-carvedilol in human liver microsomes were CYP2D6 and CYP2C9 and to a lesser extent CYP3A4, 2C19, 1A2, and 2E1.
- Carvedilol is subject to the effects of genetic polymorphism with poor metabolizers of debrisoquin (a marker for cytochrome P450 2D6) exhibiting 2- to 3-fold higher plasma concentrations of R(+)-carvedilol compared to extensive metabolizers. In contrast, plasma levels of S(-)-carvedilol are increased only about 20% to 25% in poor metabolizers, indicating this enantiomer is metabolized to a lesser extent by cytochrome P450 2D6 than R(+)-carvedilol.
- Half-Life: 7 - 10 hours
- Specific Populations:
- Heart Failure: Steady-state plasma concentrations of carvedilol and its enantiomers increased proportionally over the 6.25 to 50 mg dose range in patients with heart failure. Compared to healthy subjects, heart failure patients had increased mean AUC and Cmax values for carvedilol and its enantiomers, with up to 50% to 100% higher values observed in 6 patients with NYHA class IV heart failure. The mean apparent terminal elimination half-life for carvedilol was similar to that observed in healthy subjects.
- Geriatric: Plasma levels of carvedilol average about 50% higher in the elderly compared to young subjects.
- Hepatic Impairment: Compared to healthy subjects, patients with severe liver impairment (cirrhosis) exhibit a 4- to 7-fold increase in carvedilol levels. Carvedilol is contraindicated in patients with severe liver impairment.
- Renal Impairment: Although carvedilol is metabolized primarily by the liver, plasma concentrations of carvedilol have been reported to be increased in patients with renal impairment. Based on mean AUC data, approximately 40% to 50% higher plasma concentrations of carvedilol were observed in hypertensive patients with moderate to severe renal impairment compared to a control group of hypertensive patients with normal renal function. However, the ranges of AUC values were similar for both groups. Changes in mean peak plasma levels were less pronounced, approximately 12% to 26% higher in patients with impaired renal function.
- Consistent with its high degree of plasma protein-binding, carvedilol does not appear to be cleared significantly by hemodialysis.
- Drug-Drug Interactions: Since carvedilol undergoes substantial oxidative metabolism, the metabolism and pharmacokinetics of carvedilol may be affected by induction or inhibition of cytochrome P450 enzymes.
- Amiodarone: In a pharmacokinetic study conducted in 106 Japanese patients with heart failure, coadministration of small loading and maintenance doses of amiodarone with carvedilol resulted in at least a 2-fold increase in the steady-state trough concentrations of S(-)-carvedilol.
- Cimetidine: In a pharmacokinetic study conducted in 10 healthy male subjects, cimetidine (1,000 mg/day) increased the steady-state AUC of carvedilol by 30% with no change in Cmax.
- Digoxin: Following concomitant administration of carvedilol (25 mg once daily) and digoxin (0.25 mg once daily) for 14 days, steady-state AUC and trough concentrations of digoxin were increased by 14% and 16%, respectively, in 12 hypertensive patients.
- Glyburide: In 12 healthy subjects, combined administration of carvedilol (25 mg once daily) and a single dose of glyburide did not result in a clinically relevant pharmacokinetic interaction for either compound.
- Hydrochlorothiazide: A single oral dose of carvedilol 25 mg did not alter the pharmacokinetics of a single oral dose of hydrochlorothiazide 25 mg in 12 patients with hypertension. Likewise, hydrochlorothiazide had no effect on the pharmacokinetics of carvedilol.
- Rifampin: In a pharmacokinetic study conducted in 8 healthy male subjects, rifampin (600 mg daily for 12 days) decreased the AUC and Cmax of carvedilol by about 70%.
- Torsemide: In a study of 12 healthy subjects, combined oral administration of carvedilol 25 mg once daily and torsemide 5 mg once daily for 5 days did not result in any significant differences in their pharmacokinetics compared with administration of the drugs alone.
- Warfarin: Carvedilol (12.5 mg twice daily) did not have an effect on the steady-state prothrombin time ratios and did not alter the pharmacokinetics of R(+)- and S(-)-warfarin following concomitant administration with warfarin in 9 healthy volunteers.
- Advise patients to take carvedilol with food.
- Advise patients that they should not interrupt or discontinue using carvedilol without a physician's advice.
- Advise patients with heart failure to consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
- Advise patients that they may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
- If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
- Advise patients to consult a physician if they experience dizziness of faintness, incase the dosage should be adjusted.
- Advise diabetic patients to report any changes in blood sugar levels to their physician.
- Advise contact lens wearers that they may experience decreased lacrimation.
Indications
Dosing
(Adult):
Dosage Forms
Contraindications
Warnings
Adverse Reactions
Overdose
Antidote
Drug Interactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|