Aztreonam (Azactam; Cayston): Drug Monograph
|
|---|
- Monobactam antibiotic
- Time-Dependent, Concentration Independent Antibacterial Activity
- Bactericidal (not dependent on immune system)
- Adjunct to Surgery including abscesses, infections complicating hollow viscus perforations, cutaneous infections, and infections of serous surfaces. It is effective against most of the commonly encountered Gram-negative aerobic pathogens seen in general surgery
- Cystic Fibrosis (nebulization therapy) lung infection caused by Pseudomonas aeruginosa
- Intra-Abdominal Infections, including peritonitis caused by Escherichia coli, Klebsiella species including K. pneumoniae, Enterobacter species including E. cloacae, Pseudomonas aeruginosa, Citrobacter species including C. freundii, and Serratiaspecies including S. marcescens.
- Gynecologic Infections, including endometritis and pelvic cellulitis caused by Escherichia coli, Klebsiella pneumoniae,Enterobacter species including E. cloacae, and Proteus mirabilis.
- Lower Respiratory Tract Infections, including pneumonia and bronchitis caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacterspecies, and Serratia marcescens.
- Septicemia caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis*, Serratia marcescens, and Enterobacter species.
- Skin and Skin-Structure Infections, including those associated with postoperative wounds, ulcers, and burns, caused by Escherichia coli, Proteus mirabilis, Serratia marcescens, Enterobacter species, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Citrobacter species.
- Urinary Tract Infections (complicated and uncomplicated), including pyelonephritis and cystitis (initial and recurrent) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter species, and Serratia marcescens.
- May be administered intravenously or by intramuscular injection. Inhalation (nebulized administration)
- Cystic Fibrosis (Inhalation Solution):
- Children > 7 yrs: 75 mg by inhalation/nebulization 3 times per day x 28 days
- Inhalation is only by Altera nebulizer system. Give a short acting bronchodilator 15 minutes to 4 hours prior to dose.
- Moderately severe systemic infections:
- 1 or 2 g IV/IM every 8 or 12 hours.
- Severe systemic or life-threatening infections:
- 2 g IV/IM every 6 or 8 hours.
- Surgical Infection Prophylaxis:
- 2 g IV 30-60 minutes prior to start of surgery, repeat in 4 hours if procedure is longer.
- Urinary Tract Infection:
- 500 mg or 1 g IV/IM every 8 or 12 hours
- Pediatric Starting Dose:
- Administered intravenously to patients with normal renal function.
- Mild to moderate infections: 30 mg/kg every 8 hours.
- Moderate to severe infections: 30 mg/kg every 6 to 8 hours
- IV/IM Dosing:
- CrCl 10 and 30 mL/min: Decrease dose by 50% but at the same dosing interval
- CrCl < 10 mL/min: Give 25% of initial dose or reduce by 75% but at the same dosing interval
- Hemodialysis (HD): About 20% to 50% is removed by HD. Can give initial dose based on severity of the infection and then give 12.5% to 25% of the standard dose.
- CVVHD: Give 2 g IV loading dose then 1 g every 8 hours or 2 g IV every 12 hours.
- Nebulized or Inhalation:
- No dosage adjustment needed
- Single-dose 15 mL capacity vials for injection (Azactam; generic): 1 g/vial, 2 g/vial
- Inhalation solution (Cayston): 75 mg (84 mL) preservative free
- Hypersensitivity reactions in patients with or without prior exposure.
- Clostridium difficile-associated diarrhea (CDAD) - ranges in severity from mild diarrhea to fatal colitis
- Rare cases of toxic epidermal necrolysis in patients undergoing bone marrow transplant with multiple risk factors including sepsis, radiation therapy, and other concomitantly administered drugs associated with toxic epidermal necrolysis
- Impaired renal function
- Overgrowth of nonsusceptible organisms, including Gram-positive organisms (Staphylococcus aureus and Streptococcus faecalis) and fungi.
- IV/IM Administration:
- Local reactions - phlebitis/thrombophlebitis, discomfort and swelling at the injection site
- Diarrhea
- Nausea and/or vomiting
- Rash
- Inhalation (Nebulized) Solution:
- Cough
- Wheezing
- Sore throat
- Fever
- Pediatric patients:
- Rash
- Diarrhea
- Fever
- Pain
- Erythema
- Induration
- Phlebitis
- Pregnancy: Pregnancy Category B
- Labor and Delivery: None
- Nursing Mothers: Aztreonam is excreted in human milk in concentrations that are less than 1% of concentrations determined in simultaneously obtained maternal serum; consideration should be given to temporary discontinuation of nursing and use of formula feedings.
- Renal Impairment: None
- Hepatic Impairment: None
- Pediatric Patients: The safety and effectiveness of intravenous
aztreonam have been established in the age groups 9 months to 16 years. Sufficient data are not available for
pediatric patients under 9 months of age or for the following treatment
indications/pathogens: septicemia and
skin and skin-structure infections (where the skin infection is believed or
known to be due to H. influenzae type
b). In pediatric patients with cystic
fibrosis, higher doses may be warranted.
- Geriatric Patients: No differences in responses between the elderly and younger patients have been identified. Dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Renal function should be monitored and dosage adjustments made accordingly.
- Aztreonam is excreted in human milk in concentrations that are less than 1% of concentrations determined in simultaneously obtained maternal serum
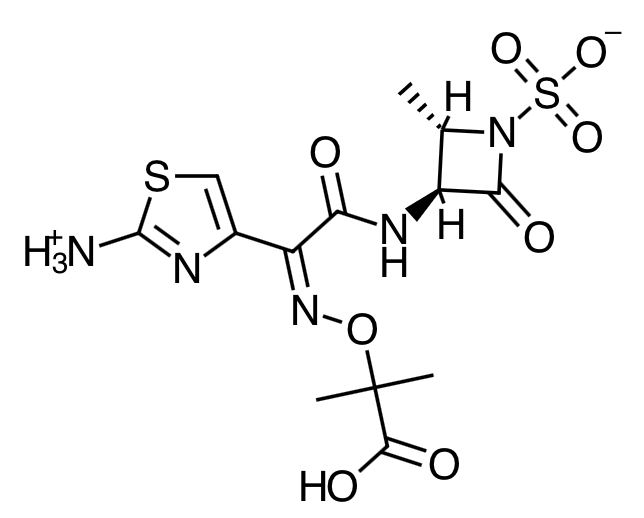
- Scientific Name: (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl- 4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid.
- Empirical Formula: C13H17N5O8S2
- Molecular Weight: 435.44
- Aztreonam is a beta-lactam antibiotic that has bactericidal activity and works by inhibition of bacterial cell wall synthesis through the inhibition of penicillin-binding protein (transpeptidase).
- It primarily kills in a time-dependent or concentration independent fashion.
- The serum levels of aztreonam following single 500 mg or 1 g (intramuscular or intravenous) or 2 g (intravenous) doses of aztreonam exceed the MIC 90 for Neisseria sp., Haemophilus influenzae, and most genera of the Enterobacteriaceae for 8 hours (for Enterobacter sp., the 8-hour serum levels exceed the MIC for 80% of strains). For Pseudomonas aeruginosa, a single 2 g intravenous dose produces serum levels that exceed the MIC 90 for approximately 4 to 6 hours. All of the above doses of aztreonam result in average urine levels of aztreonam that exceed the MIC 90 for the same pathogens for up to 12 hours.
- Absorption:
- After a single IM injection, maximum serum concentrations of 500 mg and 1 g doses occur at about 1 hour. After identical single intravenous or intramuscular doses of aztreonam, the serum concentrations of aztreonam are comparable at 1 hour (1.5 hours from start of intravenous infusion) with similar slopes of serum concentrations thereafter.
- Distribution:
- Apparent mean volume of distribution at steady-state averaged 12.6 liters
- Protein binding averaged 56% and was independent of dose
- Aztreonam given IV rapidly reaches therapeutic concentrations in peritoneal dialysis fluid; conversely, aztreonam given intraperitoneally in dialysis fluid rapidly produces therapeutic serum levels
- Metabolism: None
- Elimination:
- Half-life of aztreonam averaged 1.7 hours (1.5-2.0) with normal renal function, independent of the dose and route of administration.
- In elderly patients, the mean serum half-life of aztreonam increased and the renal clearance decreased, consistent with the age-related decrease in creatinine clearance. The dosage should be adjusted accordingly.
- Impaired renal function, the serum half-life of aztreonam is prolonged. The serum half-life of aztreonam is only slightly prolonged in patients with hepatic impairment since the liver is a minor pathway of excretion.
- Clearance was 91 mL/min and renal clearance was 56 mL/min.
- Excretion is via the urine about equally by active tubular secretion and glomerular filtration. Approximately 60% to 70% of an IV/IM dose was recovered in the urine by 8 hours. Urinary excretion of a single parenteral dose was essentially complete by 12 hours after injection. About 12% of a single intravenous radiolabeled dose was recovered in the feces.
- Patients should be counseled that antibacterial drugs, including aztreonam, should only be used to treat bacterial infection. They do not treat viral infections (e.g., the common cold).
- Patients should be told to take the medication exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the immediate treatment and increase the likelihood that bacteria will develop resistance and will not be treatable by aztreonam or other antibacterial drugs in the future.
- Patients should be informed that they may develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Class
Indications
Dosing
Renal Dosing
Dosage Forms
Warnings
Adverse Reactions
Special Populations
Breastfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
MESH Terms & Keywords
|
|---|
|