Apixaban (Eliquis): Drug Monograph
|
|---|
- Reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Deep vein thrombosis (DVT) Prevention Post-Hop or Knee Replacement
- Deep vein thrombosis (DVT) Treatment
- Pulmonary Embolism (PE)
- Stroke Prevention in Non-Valvular Atrial Fibrillation:
- 5 mg orally twice daily unless the patient meets any one of the following (if so, then reduce the dose to 2.5 mg orally twice daily):
- ≥ 80 yrs of age
- Weight ≤ 60 kg
- Serum Cr ≥ 1.5 mg/dL
- DVT Prevention (Post-Hip & Post0Knee Replacement):
- Post-Hip: 2.5 mg orally twice a day starting 12-24 hrs post-operatively x 35 days
- Post-Knee: 2.5 mg orally twice a day starting 12-24 hrs post-operatively x 12 days
- Note: Patients with serum Cr > 2.5 or CrCl < 25 mL/min were excluded from trials
- DVT Treatment:
- 10 mg orally twice a day x 7 days, then reduce to 5 mg twice daily x 6 months
- Reduce the risk of recurrence after 6 months of treatment: 2.5 mg twice daily
- Note: Patients with serum Cr > 2.5 or CrCl < 25 mL/min were excluded from trials.
- PE Treatment:
- 10 mg orally twice a day x 7 days, then reduce to 5 mg twice daily x 6 months
- Reduce the risk of recurrence after 6 months of treatment: 2.5 mg twice daily
- Note: Patients with serum Cr > 2.5 or CrCl < 25 mL/min were excluded from trials
- Converting from Warfarin à Apixaban:
- Once the INR is < 2, stop warfarin and start Apixaban
- Converting from Apixaban à Warfarin:
- Since Apixaban can increase the INR, the use of the INR to dose warfarin is initially unreliable.
- If continuous anticoagulation is needed, stop Apixaban and start a parenteral anticoagulant (e.g., heparin) along with standard doses of warfarin and continue parenteral anticoagulant until INR is in desired therapeutic range.
- For indications (DVT Prevention, DVT Treatment, and PE Treatment):
- No specific dosing recommendations however patients with serum Cr > 2.5 or CrCl < 25 mL/min were excluded from trials.
- Non-Valvular Atrial Fibrillation:
- If serum Cr is < 1.5, no adjustment needed unless the ≥ 80 yrs of age, weight ≤ 60 kg where the dose is reduced to 2.5 mg twice daily
- If serum Cr is ≥ 1.5 mg/dL (but < 2.5 mg/dL) and either ≥ 80 yrs of age or weight ≤ 60 kg reduce dose to 2.5 mg twice daily
- If ESRD on HD: 5 mg twice a day and reduce dose to 2.5 mg twice daily if ≥ 80 yrs of age, weight ≤ 60 kg.
- Discontinuing apixaban in patients without adequate continuous anticoagulation increases risk of stroke. If anticoagulation with apixaban must be discontinued for a reason other than pathological bleeding, coverage with another anticoagulant should be strongly considered.
- Epidural hematomas can occur in patients getting neuraxial anesthesia or undergoing a spinal procedure while on Apixaban.
- Apixaban can cause serious, potentially fatal bleeding. Promptly evaluate signs and symptoms of blood loss.
- Prosthetic heart valves: apixaban use not recommended.
- Substrate: CYP3A4 (major), P-gp, BCRP
- Inhibits: CYP2C19 (weak)
- Drug Interactions:
- Strong dual inhibitors of CYP3A4 and P-gp increase blood levels of apixaban; reduce apixaban dose to 2.5 mg or avoid concomitant use.
- Simultaneous use of strong inducers of CYP3A4 and P-gp reduces blood levels of apixaban. Avoid concomitant use.
- Pregnancy: Pregnancy Category B. Use of apixaban is not recommended.
- Labor and Delivery: Safety and effectiveness during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using apixaban in this setting.
- Nursing Mothers: It is unknown if apixaban or its metabolites are excreted in human milk. Women should be instructed either to discontinue breastfeeding or to discontinue apixaban therapy, taking into account the importance of the drug to the mother.
- Renal Impairment:
- Hepatic Impairment: Use of apixaban is not recommended.
- Pediatric Patients: Safety and effectiveness in pediatric patients have not been established.
- Geriatric Patients: The effects of apixaban on the risk of stroke and major bleeding compared to warfarin were maintained in geriatric subject.
- It is unknown if apixaban or its metabolites are excreted in human milk. Women should be instructed either to discontinue breastfeeding or to discontinue apixaban therapy, taking into account the importance of the drug to the mother.
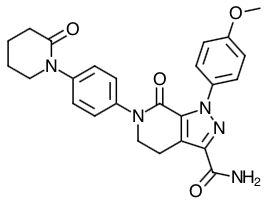
- Scientific Name: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide.
- Empirical Formula: C25H25N5O
- Molecular Weight: 459.5
- Apixaban is an oral, reversible, and selective active site inhibitor of FXa and does so without the need or presence of antithrombin III for its antithrombotic activity.
- Apixaban can inhibit both free and clot-bound FXa, and prothrombinase activity.
- While it has has no direct effect on platelet aggregation, it can indirectly inhibits platelet aggregation induced by thrombin (by inhibiting FXa, apixaban decreases thrombin generation and thrombus development).
- As a result of FXa inhibition, apixaban prolongs clotting tests such as prothrombin time (PT), INR, and activated partial thromboplastin time (aPTT). Change observed in these clotting tests at the expected therapeutic dose, however, are small, subject to a high degree of variability, and not useful in monitoring the anticoagulation effect of apixaban.
- The Rotachrom® Heparin chromogenic assay was used to measure the effect of apixaban on FXa activity in humans during the apixaban development program. A concentration-dependent increase in anti-FXa activity was observed in the dose range tested and was similar in healthy subjects and patients with AF.
- This test is not recommended for assessing the anticoagulant effect of apixaban.
- Apixaban displays prolonged absorption. Thus despite a short clearance half-life of about 6 hours, the apparent half-life during repeat dosing is about 12 hours, which allows twice-daily dosing to provide effective anticoagulation, but it also means that when the drug is stopped for surgery, anticoagulation persists for at least a day.
- Absorption: The absolute bioavailability ~ 50% for doses up to 10 mg of apixaban. Food does not affect the bioavailability of apixaban.
- Peak Absorption: Maximum concentrations (Cmax) of apixaban appear 3 to 4 hours after oral administration of apixaban. Apixaban demonstrates linear pharmacokinetics with dose-proportional increases in exposure for oral doses up to 10 mg.
- Protein Binding: Plasma protein binding in humans is approximately 87%.
- Volume of distribution (Vss): Approximately 21 liters.
- Metabolism: Approximately 25% of an orally administered apixaban dose is recovered in urine and feces as metabolites. Apixaban is metabolized mainly via CYP3A4 with minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2. O-demethylation and hydroxylation at the 3-oxopiperidinyl moiety are the major sites of biotransformation.
- Unchanged apixaban is the major drug-related component in human plasma; there are no active circulating metabolites.
- Elimination: Apixaban is eliminated in both urine and feces. Renal excretion accounts for about 27% of total clearance. Biliary and direct intestinal excretion contributes to elimination of apixaban in the feces.
- Half-life (apparent): ~12 hours because of prolonged absorption.
- Specific Populations:
- No meaningful differences between males and females in pharmacokinetics.
- The results across pharmacokinetic studies in normal subjects showed no differences in apixaban pharmacokinetics among White/Caucasian, Asian, and Black/African American subjects. No dose adjustment is required based on race/ethnicity.
- They should not discontinue apixaban without talking to their physician first.
- They should be informed that it might take longer than usual for bleeding to stop and they may bruise or bleed more easily when treated with apixaban. Advise patients about how to recognize bleeding or symptoms of hypovolemia and or the urgent need to report any unusual bleeding to their physician.
- They should tell their physicians and dentists they are taking apixaban, and/or any other product know to affect bleeding (including nonprescription products, such as aspirin or NSAIDs), before any surgery or medical or dental procedure is scheduled and before any new drug is taken.
- They should tell their physicians if they are pregnant or plan to become pregnant or are breastfeeding or intends to breastfeed during treatment with apixaban.
- If a dose is missed, the dose should be taken as soon as possible on the same day and twice daily administration should be resumed. The dose should not be doubled to make up for a missed dose.
Indications
Dosing
Note: Dose adjustments considered if serum Cr > 1.5, weight is < 60 kg, or patient is taking other medications known to be strong inhibitors of CYP3A4 and P-glycoprotein
Renal Dosing
Black Box Warnings
Warnings
Drug Interactions
Special Populations
Breasfeeding
Chemical Structure
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
Counseling Points
Advise patients of the following:
MESH Terms & Keywords
|
|---|
|

